A groundbreaking collaboration involving four groups at MIT, under the leadership of principal investigators Laura L. Kiessling, Jeremiah A. Johnson, Alex K. Shalek, and Darrell J. Irvine, alongside researchers at Georgia Tech led by M.G. Finn, has unveiled an innovative method for activating the immune system against cancer cells. This research, published in ACS Nano, opens new avenues for developing tumor vaccines that enhance anti-tumor immunity both preventively and therapeutically.
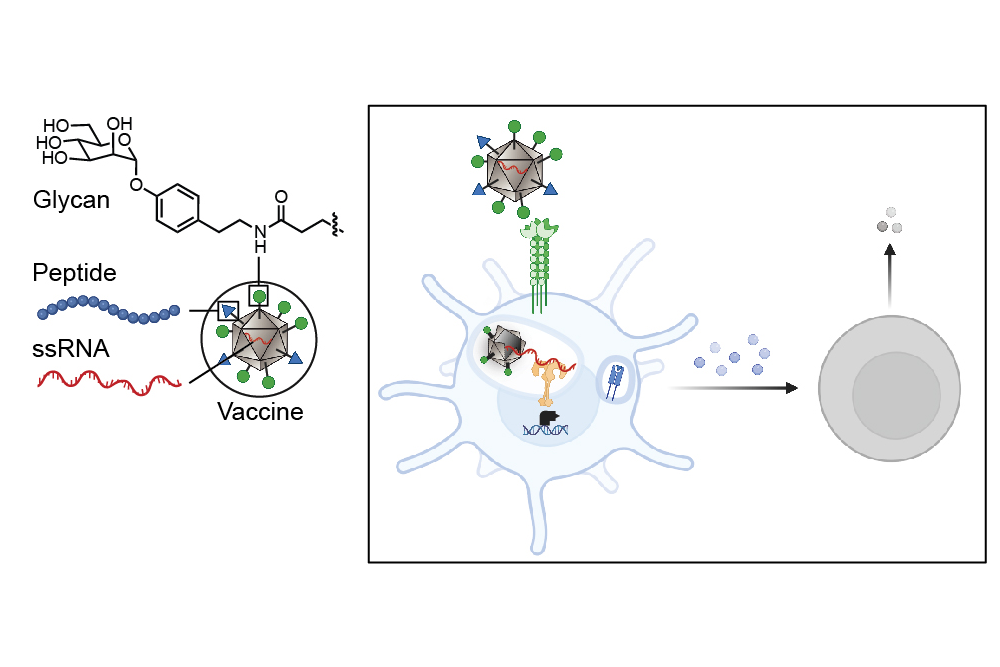
Cancer cells often resemble the healthy cells from which they originate, making it challenging for the immune system to recognize them as threats. Unlike these cells, pathogens such as viruses, bacteria, and fungi possess unique carbohydrates that distinctly differ from human carbohydrates. Dendritic cells, which are the immune system’s most effective antigen-presenting cells, recognize these unconventional carbohydrates and process the corresponding antigens to alert the immune system. This study introduces a method to target these antigens to dendritic cells, resulting in a more robust immune response.
Overcoming Tumor Resistance
The researchers’ approach involves cloaking tumor antigens in foreign carbohydrates while also co-delivering them with single-stranded RNA, enabling dendritic cells to identify these tumor proteins as threats. By targeting the lectin DC-SIGN, recognized for its role in enhancing dendritic cell immune responses, the team decorated a virus-like particle—an assembly of virus proteins and non-infectious RNA—with DC-SIGN-binding carbohydrate derivatives. As a result, these glycan-coated virus-like particles exhibit unique sugars, prompting dendritic cells to mount an attack.
“Dendritic cells have carbohydrate-binding proteins known as lectins that bind to sugars on the surfaces of viruses and bacteria. This binding action helps facilitate the internalization of particles,” explains Kiessling, the senior author of the study. “Upon entry, the virus-like particle disassembles and releases its RNA.” Notably, both toll-like receptors (bound to RNA) and DC-SIGN (linked to the sugar decoration) work together to signal an immune response.
Once alert, dendritic cells initiate a vigorous immune response, significantly more potent than what typical untargeted vaccines provoke. When these cells detect antigens, they communicate with T cells, orchestrating diverse immune responses based on the activated pathways.
Innovating Cancer Vaccine Development
This new research marks a pivotal step in creating a cancer vaccine with a dual action: the vaccine’s glycan coat binds to lectins for initial signaling, while interaction with toll-like receptors promotes a vigorous immune activation.
The Kiessling, Finn, and Johnson teams had previously established a synthetic DC-SIGN binding group that successfully directed immune responses when decorating virus-like particles. However, the potential for a cancer vaccine application was previously uncertain. The collaborative efforts between MIT and Georgia Tech confirmed its effectiveness.
Valerie Lensch, a chemistry PhD student from MIT’s Program in Polymers and Soft Matter, expanded upon the existing strategy to evaluate its potential as an anticancer vaccine, deepening her understanding of immunology in the process.
“We’ve developed a modular vaccine platform aimed at stimulating antigen-specific cellular immune responses,” says Lensch. “This platform holds promise, not just in the fight against cancer, but also for addressing challenging intracellular pathogens like malaria, HIV, and Mycobacterium tuberculosis, representing significant strides in vaccine innovation.”
After numerous in vitro iterations with the glycan-coated virus-like particles, the researchers pinpointed a promising design, advancing them to in vivo models—an exciting milestone for their work.
Adele Gabba, a postdoc in the Kiessling Lab, partnered with Lensch, while Robert Hincapie, who completed his PhD under Professor M.G. Finn at Georgia Tech, constructed and decorated the particle with glycans devised by the MIT team.
“We’re uncovering that carbohydrates function as a communication language between cells that guides the immune system,” states Gabba. “It’s exhilarating to begin decoding this language to reshape immune responses.”
“The foundational principles of this vaccine design stem from thorough research carried out by former graduate students and postdoctoral researchers over the years, focusing on optimizing lectin interactions and comprehending lectins’ role in immunity,” adds Lensch. “Witnessing the translation of these concepts into therapeutic platforms has been thrilling across various applications.”
Photo credit & article inspired by: Massachusetts Institute of Technology