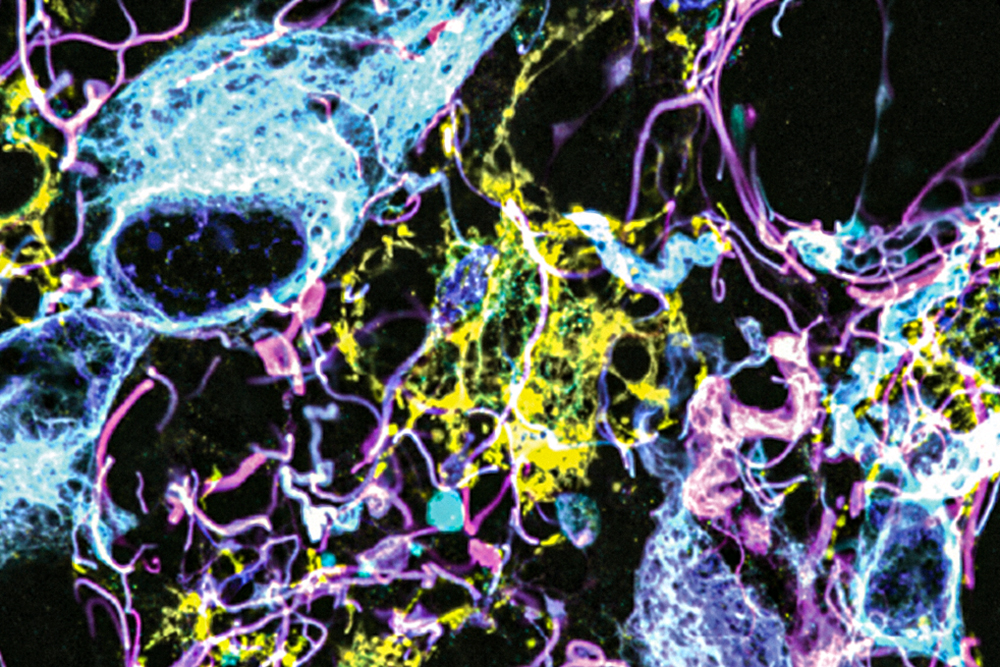
A groundbreaking microscopy technique developed by researchers from MIT and Brigham and Women’s Hospital/Harvard Medical School has allowed for unprecedented imaging of human brain tissue. This innovative approach unveils previously invisible cells and structures within the brain.
Specifically, the research revealed that certain “low-grade” brain tumors harbor a higher-than-anticipated number of potentially aggressive tumor cells, hinting that these tumors might be more aggressive than previously believed.
The team envisions that this advanced technique could pave the way for improved tumor diagnosis, more accurate prognoses, and informed treatment options for patients.
“We are beginning to understand the critical interactions between neurons, synapses, and the surrounding brain tissue that influence tumor growth and progression. Until now, these interactions were largely obscured by conventional imaging tools, but our method allows us to explore these tissues at a nanoscale level,” explains Pablo Valdes, a former MIT postdoc and current assistant professor of neuroscience at the University of Texas Medical Branch and the lead author of the study.
The study features prominent researchers such as Edward Boyden, MIT’s Y. Eva Tan Professor in Neurotechnology, and E. Antonio Chiocca, a neurosurgery professor at Harvard Medical School. Their findings are published in Science Translational Medicine today. You can view the study here.
Revolutionizing Imaging Techniques
The revolutionary imaging method leverages expansion microscopy, a technique pioneered in Boyden’s lab in 2015. The idea is straightforward: instead of relying on costly microscopes to capture high-resolution images, researchers have developed a way to physically expand the tissue being studied, making it possible to achieve exceptional detail using standard light microscopes.
This method involves embedding the tissue in a polymer that swells upon adding water, effectively softening and separating the proteins that typically hold the tissue together. By expanding the polymer with water, researchers can achieve imaging resolutions around 70 nanometers, a feat previously reserved for much more sophisticated and expensive equipment like scanning electron microscopes.
Although the Boyden lab first figured out a method for expanding preserved human tissue in 2017, the agents used in that process often damaged critical proteins. For this study, the authors developed a novel protocol to soften the tissue while preserving its proteins, allowing for enhanced imaging with fluorescent antibodies.
“Our approach opens up space between proteins, enabling antibodies to access regions they couldn’t previously reach,” Valdes notes. “With tissue expansion, we can visualize numerous proteins in a single sample, enhancing our understanding of their relationships.”
Collaborating with MIT Assistant Professor Deblina Sarkar, the team demonstrated this innovative “decrowding” approach using mouse tissue in 2022. The current study successfully applied this technique to human brain samples used in clinical diagnostics, a greater challenge due to the treatment processes these tissues often undergo.
In this research, the team labeled as many as 16 different molecules per tissue sample, targeting various structures and cell types, including axons, synapses, astrocytes, and blood vessel cells. They also studied proteins associated with tumor aggression and neurodegeneration.
By examining healthy brain tissue alongside samples from patients with two glioma types—high-grade glioblastoma, known for its aggressiveness and poor prognosis, and low-grade gliomas, identified as less aggressive—the researchers aimed to deepen the understanding of these tumors at a nanoscale.
“Our goal was to investigate brain tumors comprehensively to enhance our understanding at a nanoscale level, ultimately leading to improved treatment options and diagnoses. The field of neuro-oncology has been slow to adopt super-resolution imaging techniques,” states Valdes.
Enhancing Diagnostic Capabilities
To differentiate aggressive tumor cells within gliomas, the researchers used vimentin, a protein commonly found in aggressive glioblastomas. The results were eye-opening, revealing a significantly higher number of vimentin-expressing tumor cells in low-grade gliomas than previously detected by standard methods.
“This finding provides new insights into the biology of these tumors, indicating some may have a more aggressive nature than is typically recognized through conventional staining techniques,” Valdes adds.
Current surgical procedures for glioma patients typically involve preserving tumor samples for analysis using immunohistochemistry staining. This method can reveal certain markers indicating tumor aggressiveness, including several discussed in this study.
“Given the incurable nature of these brain cancers, our discoveries could assist in identifying pivotal cancer molecules to target for designing enhanced treatments,” says Chiocca. “This underscores the collaborative impact of clinicians and basic scientists, such as Ed Boyden, conveniently united at Brigham and Women’s Hospital and MIT.”
The researchers aspire that their expansion microscopy technique will unlock deeper insights into patients’ tumors, leading to better assessments of tumor aggression and improved treatment strategies. Valdes is already planning larger-scale studies on various tumor types to establish diagnostic guidelines based on traits revealed by this technique.
“We envisage this as a diagnostic tool to identify significant marker cells and interactions that were previously undetectable,” he expresses. “This straightforward methodology will provide an unprecedented view into neurological diseases at the nanoscale, facilitating advancements in neuro-oncology and neuropathology.”
Boyden’s lab also intends to expand this technique’s application to study other facets of brain function, both in healthy conditions and various diseases.
“The ability to conduct nanoimaging is vital, as biology fundamentally revolves around nanoscale components—genes, biomolecules—that interact over minuscule distances,” Boyden highlights. “We can examine numerous nanoscale interactions, including those influencing synaptic changes, immune responses, and transformations occurring during cancer and aging.”
This research received generous support from K. Lisa Yang, the Howard Hughes Medical Institute, John Doerr, Open Philanthropy, the Bill and Melinda Gates Foundation, the Koch Institute Frontier Research Program via the Kathy and Curt Marble Cancer Research Fund, the National Institutes of Health, and the Neurosurgery Research and Education Foundation.
Photo credit & article inspired by: Massachusetts Institute of Technology