Our immune system is like a vigilant guard, tasked with identifying and eliminating cells that have undergone cancerous mutations. Yet, certain early-stage cancer cells have developed clever tactics to slip under this immune radar and progress into more advanced tumors.
A groundbreaking study conducted by researchers from MIT and Dana-Farber Cancer Institute has uncovered a significant strategy that enables these precancerous cells to evade immune detection. This research highlights the role of a gene known as SOX17, which, when activated in early colon cancer development, allows these cells to become effectively invisible to the immune response.
If researchers can find methods to inhibit the function of SOX17 or its associated pathways, it could pave the way for innovative treatments targeting early-stage cancers, potentially halting their progression into larger tumors, according to the study’s findings.
“The activation of the SOX17 pathway in the initial stages of colorectal cancer is crucial, as it provides a shield for precancerous cells against the immune system. By inhibiting the SOX17 pathway, we may improve our ability to prevent colon cancer, particularly in patients susceptible to developing colon polyps,” explains Omer Yilmaz, associate professor of biology at MIT and senior author of the study.
Judith Agudo, a principal investigator at Dana-Farber Cancer Institute and a Harvard Medical School assistant professor, also played a key role in this study, published in Nature. The lead author is MIT Research Scientist Norihiro Goto, along with collaborators Tyler Jacks, a professor at MIT’s Koch Institute, and others.
Understanding Immune Evasion
Colon cancer typically originates in long-lived intestinal stem cells, responsible for regenerating the intestinal lining. Over time, these cells can accumulate mutations leading to the formation of polyps, which are premalignant growths that may develop into metastatic colon cancer.
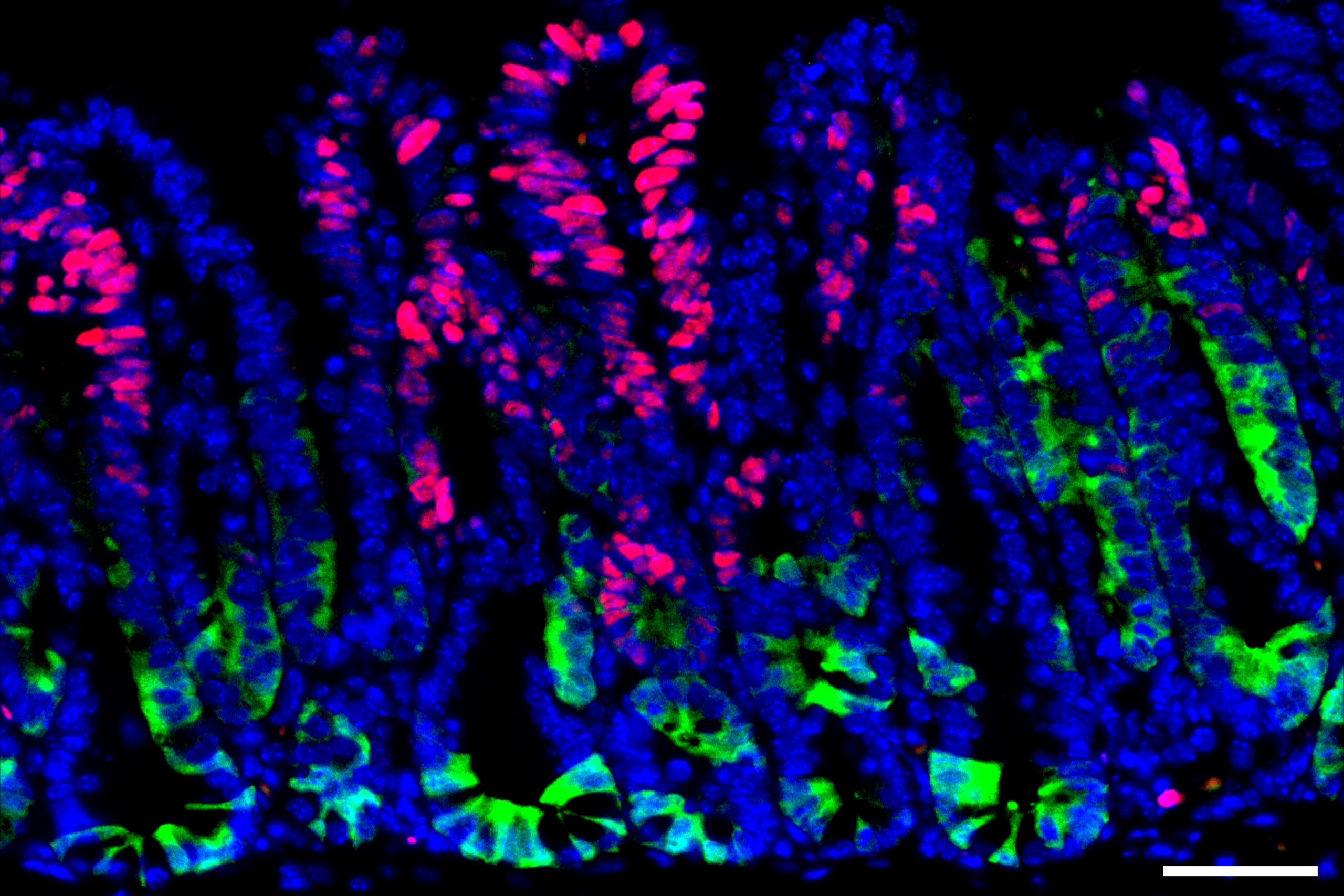
To delve deeper into how these precancerous growths dodge immune detection, the researchers employed a method they previously developed for cultivating mini colon tumors in lab dishes and subsequently implanting them into mice. They engineered these tumors to express mutated versions of cancer-associated genes such as Kras, p53, and APC, which are frequently found in human colon cancers.
After implanting these tumors, a striking increase in the expression of SOX17 was observed. This gene encodes a transcription factor active mainly during embryonic development, guiding the growth of the intestines and blood vessels.
Through their experiments, the researchers discovered that SOX17 activation in cancer cells fosters an immunosuppressive environment. Specifically, it inhibits the production of receptors that typically detect interferon gamma, an integral component of the immune system’s defense against cancer.
Without these crucial receptors, cancerous and precancerous cells can effectively disregard signals from the immune system that would normally prompt them toward apoptosis, or programmed cell death.
“SOX17 primarily functions to deactivate the interferon gamma signaling pathway in colorectal cancer cells and precancerous adenoma cells, effectively rendering the tumor cells hidden from T cells, allowing them to proliferate even in the presence of the immune system,” remarks Yilmaz.
Moreover, the absence of interferon gamma signaling diminishes cancer cells’ ability to produce MHC proteins, which are essential for presenting cancerous antigens to the immune system. This insensitivity also hampers the secretion of chemokines, which typically attract T cells to eliminate cancerous cells.
Targeting SOX17 for Treatment
The research team found that when they created colon tumor organoids with SOX17 knocked out and implanted them into mice, the immune system was far more successful in targeting these tumors. This suggests that inhibiting the activity of SOX17 could be a viable strategy for treating colon cancer in its early phases.
“By disabling SOX17 in relatively complex tumors, we were able to substantially diminish the survival capacity of these tumor cells,” Goto states.
In their study, the researchers also scrutinized gene expression data from colon cancer patients, discovering that SOX17 is often highly expressed in early-stage colon cancers but decreases as tumors become more aggressive and invasive.
“This trend is logical since, as colorectal cancers advance and spread, they activate alternative mechanisms that promote an immunosuppressive environment, reducing the dependence on SOX17,” Yilmaz explains.
While targeting transcription factors like SOX17 can be challenging due to their complex structures, the researchers aim to identify other proteins interacting with SOX17 to explore simpler potential targets for blocking these interactions.
Additionally, they are investigating the triggers that activate SOX17 in precancerous cells.
This research has received support from the MIT Stem Cell Initiative via Fondation MIT, the National Institutes of Health/National Cancer Institute, and a Koch Institute-Dana Farber Harvard Cancer Center Bridge Project grant.
Photo credit & article inspired by: Massachusetts Institute of Technology