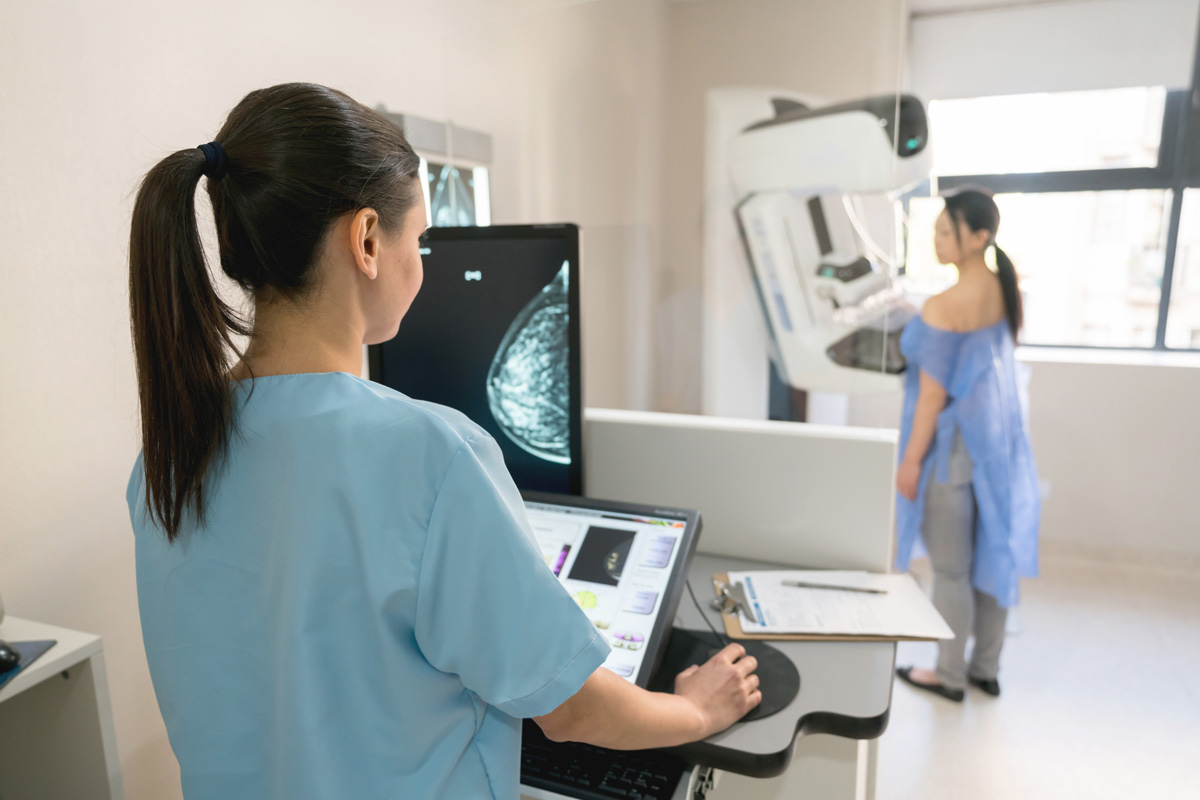
Ductal carcinoma in situ (DCIS), a preinvasive tumor, has the potential to advance to an aggressive form of breast cancer, making up approximately 25% of breast cancer cases. Despite its prevalence, DCIS poses a challenge for clinicians due to the difficulties in accurately determining its type and stage, often resulting in overtreatment of patients.
To combat this issue, a multidisciplinary team from MIT and ETH Zurich has engineered an innovative AI model that can assess various stages of DCIS using simple and cost-effective breast tissue images. This groundbreaking research emphasizes the importance of not just the cellular state, but also the spatial arrangement of cells in the tissue samples for accurately determining the stage of DCIS.
The accessibility of these tissue images enabled the researchers to compile one of the largest datasets in this domain, which was then utilized to train and validate their model. Their findings revealed a strong correlation between the AI model’s predictions and the assessments made by trained pathologists.
This AI model holds promise as a valuable tool for health professionals, potentially simplifying the diagnosis of straightforward cases and allowing clinicians to focus their effort on more complex instances of DCIS that may evolve into invasive cancer. “We’ve pioneered an approach that considers the spatial configuration of cells when evaluating DCIS. Our scalable technique sets the stage for subsequent studies. Collaborating with hospitals to bring this model into clinical practice will be a significant advancement,” explains Caroline Uhler, a professor in MIT’s Electrical Engineering and Computer Science Department and the director of the Eric and Wendy Schmidt Center at the Broad Institute of MIT and Harvard.
Uhler, who is co-corresponding author on the research paper, is joined by lead author Xinyi Zhang, a graduate student at MIT, co-corresponding author GV Shivashankar from ETH Zurich, as well as other contributors from MIT, ETH Zurich, and the University of Palermo in Italy. The open-access findings were published in Nature Communications on July 20.
Integrating Imaging Techniques with AI
While 30 to 50 percent of DCIS patients can develop into invasive cancer, specific biomarkers that could predict the progression of these tumors remain elusive. Traditional methods such as multiplexed staining and single-cell RNA sequencing, despite their effectiveness, are too expensive for routine application.
In prior research, the team established that chromatin staining, a much less costly imaging method, could yield insights comparable to the pricier single-cell RNA sequencing. By marrying this economical imaging technique with a sophisticated machine-learning model, they aimed to replicate the diagnostic power of these more expensive assessments.
Their investigation began by compiling a dataset of 560 tissue images from 122 patients, encompassing three distinct DCIS stages. This dataset served as the training ground for the AI model, which learns to interpret the cellular conditions within the images to deduce a patient’s cancer stage.
However, not all cells provide relevant cancer indicators; hence, the researchers designed the model to aggregate these cells meaningfully. They identified eight critical cellular states that serve as significant markers of DCIS, determining the ratios of these states present in the analyzed tissue samples.
The Importance of Cellular Organization
Changes in cellular organization also play a crucial role in cancer development. “While the proportions of various cell states are essential, understanding their organization within the sample is equally critical,” notes Shivashankar.
By incorporating both the proportions and spatial arrangements of these cells into the model, the accuracy of the AI diagnostic tool markedly improved. “It was fascinating to see how significantly spatial organization impacts our findings. Earlier research highlighted the relevance of cells near breast ducts, but the relationships between nearby cells are also vital,” emphasizes Zhang.
The model’s predictions were often in alignment with pathologists’ evaluations. In cases with ambiguous diagnoses, the model offered crucial insights into tissue features, providing valuable information for clinicians regarding cell organization to enhance decision-making.
This adaptive model has potential applications beyond just DCIS, with possibilities to be tailored for other cancer types and neurodegenerative diseases, an area currently under investigation by the researchers. “Our research demonstrates that with advanced AI techniques, even simple stains can render significant insights. There remains a vast landscape of research ahead, particularly in understanding cell organization,” Uhler concludes.
This research received support from various organizations, including the Eric and Wendy Schmidt Center at the Broad Institute, ETH Zurich, the Paul Scherrer Institute, the Swiss National Science Foundation, the U.S. National Institutes of Health, the U.S. Office of Naval Research, the MIT Jameel Clinic for Machine Learning and Health, and the MIT-IBM Watson AI Lab.
Photo credit & article inspired by: Massachusetts Institute of Technology