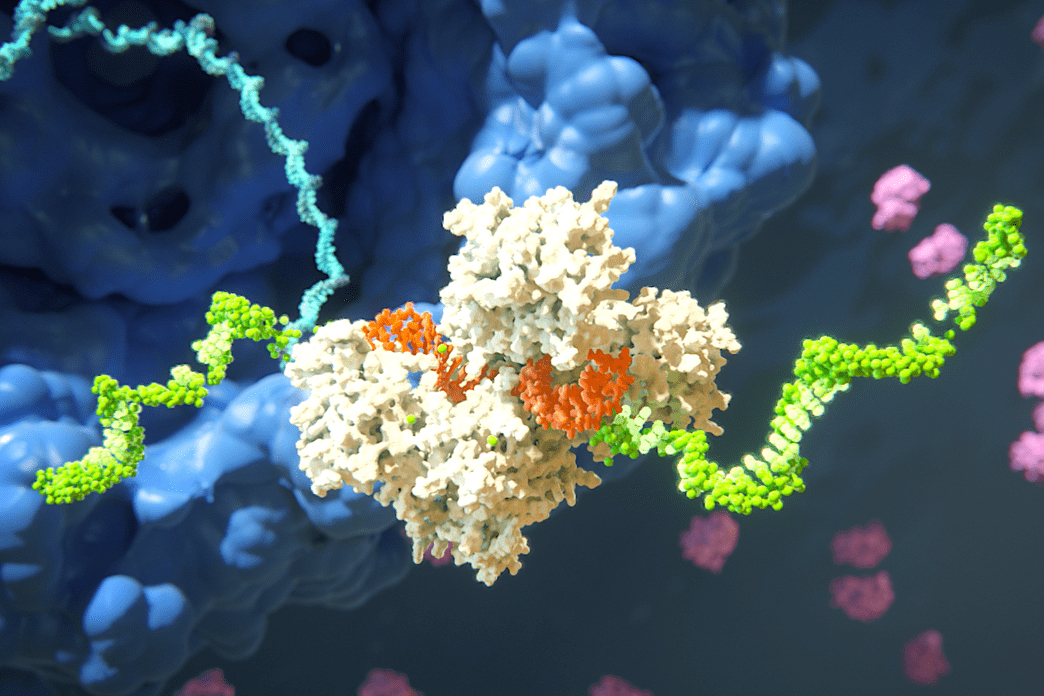
Transforming groundbreaking research into life-saving treatments is no easy feat, and the journey becomes even more complex when it involves pioneering new classes of medicine. However, conquering these challenges has the potential to revolutionize disease treatment.
Alnylam Pharmaceuticals stands as a prime example of this transformative process. Founded by a group of renowned MIT researchers, the company emerged from the ambition to leverage the revolutionary technology known as RNA interference (RNAi).
Armed with foundational research on RNAi — a natural mechanism that silences genes by degrading messenger RNA — these scholars took a significant leap in 2002 by establishing Alnylam. This pivotal decision opened the doors to crucial funding and expertise, facilitating the translation of their scientific discoveries into a new wave of medicines. Since its inception, Alnylam has successfully progressed from theoretical research to developing impactful therapeutic options.
Today, Alnylam boasts five FDA-approved medicines, with one RNAi therapeutic licensed to Novartis, alongside a rapidly developing clinical pipeline. Their approved treatments address devastating, often terminal conditions that have previously left patients with limited alternatives.
In 2023 alone, Alnylam’s therapies reached over 5,000 patients, each with stories echoing how these innovations have transformed lives. A mother of three recounted how Alnylam’s treatments empowered her to reclaim her life after suffering debilitating attacks from acute intermittent porphyria (AIP), a rare genetic disorder. Another patient shared how a treatment enabled her to attend her daughter’s wedding, while yet another successfully returned to college after dropping out due to frequent AIP episodes.
While Alnylam is not alone in RNAi medicine development, it remains a frontrunner in the field. The company’s founders — MIT Institute Professor Phil Sharp, Professor David Bartel, Professor Emeritus Paul Schimmel, along with former MIT postdocs Thomas Tuschl and Phillip Zamore — view Alnylam as a beacon for RNAi research and application.
“Alnylam has contributed over 250 scientific papers in two decades,” asserts Sharp, who leads Alnylam’s scientific advisory board. “We’ve not only conducted the science and translated it into therapies for patients, but we’ve meticulously detailed every step. We established RNAi as a treatment modality, and that’s a record I take great pride in.”
Leading RNAi Innovation
The foundation of MIT’s RNAi involvement stretches back to its very discovery. Before Andrew Fire, PhD ’83, shared the Nobel Prize for identifying RNAi in 1998, he explored the mechanisms of DNA transcription into RNA in Sharp’s laboratory.
Following his tenure at MIT, Fire and his colleagues demonstrated that double-stranded RNA could effectively silence specific genes in worms. However, the underlying biochemical mechanisms of this process remained unclear until Sharp, Bartel, Lehmann, Zamore, and Tuschl published landmark papers elucidating RNAi. They crafted a framework for studying RNAi and controlled its functionality with various genetic sequences, paving the way for investigating gene silencing in human cells.
“Tom showcased the ability to synthesize small RNAs, introduce them into cells, and achieve specific gene knockdown, which revolutionized biological research,” Bartel explains. “The prospect of precisely knocking down any mammalian gene was groundbreaking, allowing researchers to investigate the functions of genes in mammalian cells.”
Yet the implications of this breakthrough didn’t stop at enhancing gene function understanding. “With a relation between diseases and genes, I speculated, could we silence genes to treat patients?” Sharp remembers questioning.
This inquiry led to Alnylam’s formation in 2002, with the inclusion of biotech veteran Schimmel. However, significant hurdles remained in deploying this technology for patient treatment, particularly surrounding efficient RNAi delivery into patient cells.
As Alnylam Chief Scientific Officer Kevin Fitzgerald highlights, “From early findings, it became clear that three key challenges needed addressing: delivery, delivery, and delivery.” In their pursuit, Alnylam joined forces with MIT’s drug delivery specialist Bob Langer, advancing to develop the first lipid nanoparticles (LNPs) capable of encasing RNA for cellular delivery. Interestingly, these LNPs were later leveraged in COVID-19 mRNA vaccines.
“Over two decades, Alnylam has dedicated more than $4 billion to refining RNAi therapeutic developments,” Sharp notes, “This investment is crucial for translating innovations into societal benefits.”
Bringing Scientific Breakthroughs to Patients
Alnylam secured its inaugural FDA approval in 2018 for addressing hereditary transthyretin-mediated amyloidosis’ polyneuropathy—a rare, often fatal condition—marking it as the first RNAi therapeutic to enter the market and the initial drug sanctioned for this disease in the U.S.
“For some patients, two months can be a game-changer,” Fitzgerald asserts. “Given that these diseases can rapidly progress, getting a patient some relief can be monumental.”
Since that first approval, Alnylam has advanced its RNAi delivery systems — including small interfering RNA conjugation to facilitate cellular entry — and has gained FDA approvals for additional rare genetic diseases as well as high cholesterol (its treatment licensed to Novartis). Primarily targeting liver proteins’ production through gene silencing has emerged as the most feasible initial avenue; yet Alnylam’s team is optimistic about extending RNAi delivery across other body regions, unlocking a plethora of potential treatments. Promising results have emerged from trials focused on the central nervous system, with one phase one study marking the first RNAi therapeutic to achieve gene silencing in the human brain.
“Innovative research at Alnylam and concurrent efforts across the industry strive to refine RNAi delivery into various tissues: including muscles, immune, and lung cells,” Sharp comments. “However, targeting the brain stands as the most intriguing frontier; we believe this therapeutic approach can precisely regulate select genes in the nervous system, holding immense implications for conditions such as Alzheimer’s, schizophrenia, and depression.”
This CNS work resonates deeply with Fitzgerald, having witnessed his father’s struggles with Parkinson’s disease. “Our vision encompasses reaching every organ within the human body, considering combinations of organs, and targeting multiple genes within these organs,” Fitzgerald emphasizes. “We are truly just at the onset of what this technology can achieve for health outcomes.”
Currently, the RNAi scientific community is experiencing a period of dynamic growth, with ongoing studies at MIT. However, Alnylam’s successful execution of its drug development strategies remains essential to manifest the promise of these therapies and enhance patient outcomes.
“This represents a real frontier with significant therapeutic needs, and we believe this technology could massively impact health. We need to validate its efficacy, which is the core reason Alnylam exists: to probe new science, unlock new opportunities, and determine their practical application. This mission aligns perfectly with MIT’s goal to enhance lives,” Sharp concludes.
Photo credit & article inspired by: Massachusetts Institute of Technology