Intermittent fasting and low-calorie diets have gained attention for their remarkable health benefits, including delaying age-related diseases and potentially extending lifespan across various species, including humans.
A critical area of exploration stems from research conducted at MIT, revealing that fasting boosts the regenerative capabilities of intestinal stem cells, enabling better recovery from injury and inflammation.
In a groundbreaking study involving mice, MIT researchers have pinpointed the pathway that enhances this regeneration, which becomes activated when the mice transition from a fasting state to refeeding. However, there is a cautionary finding: the occurrence of cancerous mutations during this regenerative phase increases the risk of developing early-stage intestinal tumors.
“While increased stem cell activity facilitates regeneration, excessive stimulation over time may lead to adverse effects,” explains Omer Yilmaz, an associate professor of biology at MIT and senior author of the study. Though promising, further investigation is necessary to determine if these findings apply to humans.
“Our research indicates that the stages of fasting and refeeding when exposed to mutagens significantly influence cancer risk in these specific mouse models,” Yilmaz adds.
Lead authors of the study include MIT postdocs Shinya Imada and Saleh Khawaled, whose work is published in Nature.
Understanding the Drivers of Regeneration
For years, Yilmaz’s team has delved into how fasting and reduced-calorie diets impact intestinal health. A 2018 study highlighted that during fasting, intestinal stem cells shift to utilizing lipids as an energy source, enhancing their regenerative capacity. Yet, vital questions lingered: what initiates this regenerative boost during fasting, and when does it occur?
“We’ve focused on discerning whether the act of fasting or the subsequent refeeding drives this regeneration,” says Yilmaz. The new research identifies that while stem cell regeneration diminishes during fasting, it surges during refeeding.
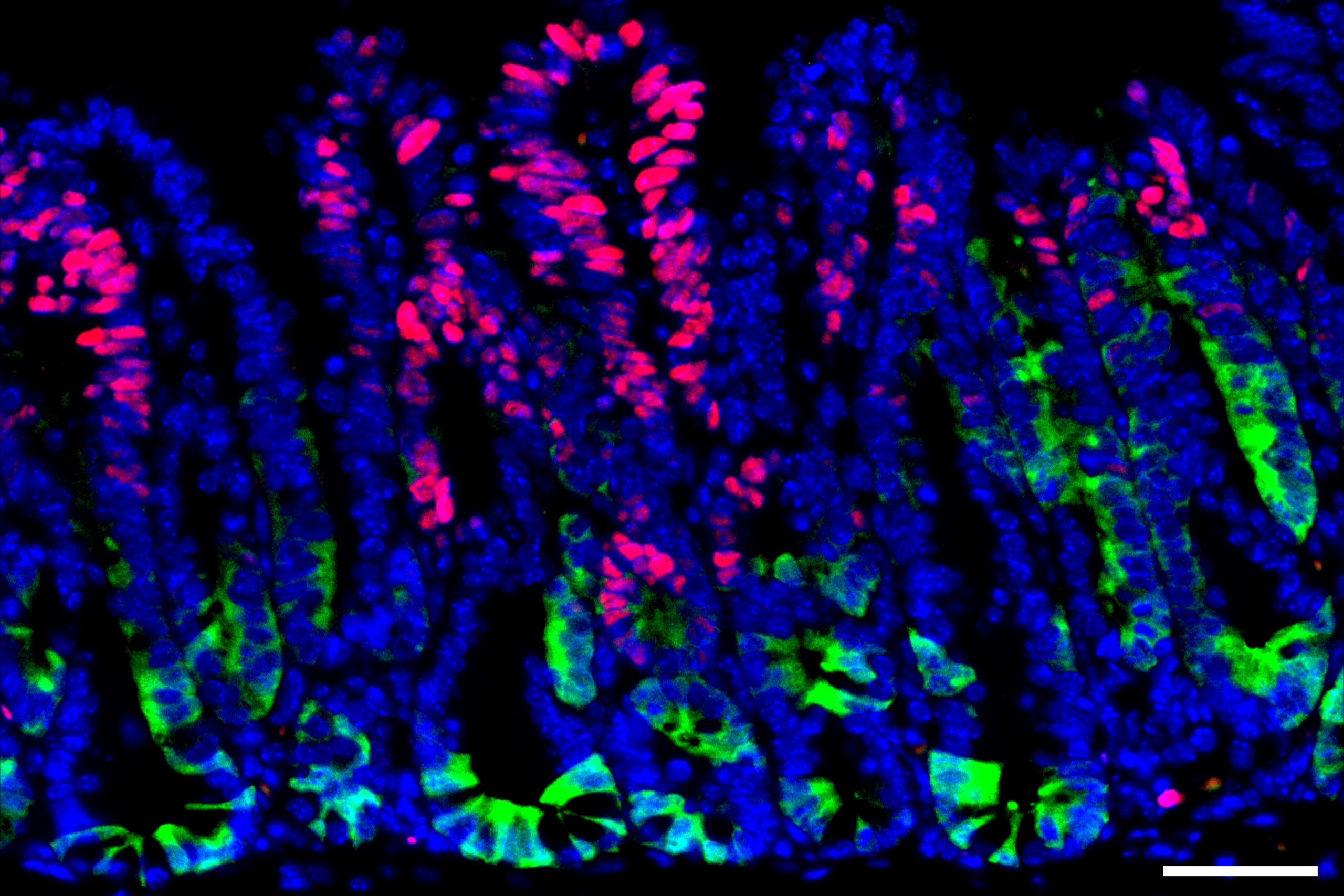
The experiment involved three groups of mice: one that fasted for 24 hours, another that fasted then re-fed for 24 hours, and a control group that continued eating normally. Observations showed that stem cells exhibited the highest proliferation levels at the end of the refeeding period, surpassing those of unfed mice.
“Fasting and refeeding represent two distinct physiological states,” Imada adds. “Fasting enhances the ability of cells to utilize lipids and fatty acids for energy, which aids survival in nutrient-scarce conditions. In contrast, the refeeding state activates processes in stem cells to proliferate and rebuild the intestinal lining.”
Further analysis revealed that these cells engage a cellular signaling pathway known as mTOR, crucial for regulating cell growth and metabolism. mTOR activation leads to increased protein synthesis essential for stem cell proliferation and creates larger quantities of polyamines—small molecules that facilitate cell growth and division.
“In the refeeding state, enhanced cell proliferation demands new cellular mass, and stem cells must generate new specialized cells to line the intestine,” explains Khawaled.
The Dangers of Over-Regeneration
Interestingly, the research revealed that while stem cells are highly regenerative, they also become more susceptible to cancerous changes. As some of the body’s most frequently dividing cells, intestinal stem cells play a pivotal role in replacing the intestinal lining every 5 to 10 days and are thus common sources of precancerous cells.
The study found that activating cancer-causing genes during the refeeding stage significantly increased the likelihood of developing precancerous polyps, compared to activation during the fasting phase. Mutations occurring during refeeding also produced a greater tendency for polyps than in consistent fed mice.
“It’s crucial to note that this research was conducted in mice with explicitly defined cancer mutations. The complexity of human responses may differ,” states Yilmaz. He cautions that while fasting has numerous health benefits, intermittent periods of refeeding right after fasting, especially when paired with mutagen exposure—like consuming charred meats—could heighten cancer risks.
The regenerative powers attributed to fasting may also serve as significant aids for individuals undergoing radiation therapy, which often compromises the intestinal lining. Yilmaz’s lab is currently exploring whether polyamine supplementation can stimulate regeneration effectively, potentially without the need for fasting.
“This compelling research sheds light on the intricate relationship between dietary habits, stem cell dynamics, and cancer risk,” remarks Ophir Klein, a professor at the University of California, San Francisco, and Cedars-Sinai Medical Center. “This study serves as a stepping stone for future testing of polyamines as potential agents to enhance intestinal repair post-injury and underscores the need for tailored dietary strategies that consider cancer risk.”
This study received funding from several sources, including the Pew-Stewart Scholars Program for Cancer Research, the MIT Stem Cell Initiative, and the Koch Institute Frontier Research Program via the Kathy and Curt Marble Cancer Research Fund.
Photo credit & article inspired by: Massachusetts Institute of Technology