MIT engineers have developed a groundbreaking tiny battery that could pave the way for cell-sized, autonomous robots capable of delivering drugs within the human body. This innovative technology also has potential applications in detecting leaks in gas pipelines.
Measuring just 0.1 millimeters in length and 0.002 millimeters in thickness—about the width of a human hair—this new battery captures oxygen from the air and utilizes it to oxidize zinc, generating a current with a potential of up to 1 volt. This output is sufficient to power small circuits, sensors, and actuators, as demonstrated by the researchers.
Michael Strano, the Carbon P. Dubbs Professor of Chemical Engineering at MIT and senior author of the study, states, “We believe this will enable significant advancements in robotics. We are integrating robotic functions into the battery, assembling various components into functional devices.”
Ge Zhang, PhD ’22, and Sungyun Yang, a graduate student at MIT, lead the research published in Science Robotics. Read the full paper here.
Revolutionizing Battery Technology
For several years, Strano’s lab has focused on creating tiny robots that can interact with and respond to environmental stimuli. A significant hurdle in advancing these miniature robots is ensuring they have an adequate and reliable power source.
While other researchers have successfully powered microscale devices using solar energy, the challenge lies in the need for a continuous light source, such as a laser, which restricts the robot’s movement. These types of devices, often referred to as “marionettes,” depend entirely on external power sources. A compact battery integrated into these devices could offer enhanced mobility and autonomy.
“Marionette systems function effectively without a built-in battery because they draw all their energy from the outside,” explains Strano. “However, for a small robot to explore previously inaccessible areas, it requires a higher degree of autonomy. Having an onboard battery is crucial for independence from external controls.”
To achieve greater autonomy in their robots, Strano’s team opted for a zinc-air battery. Renowned for their longevity and high energy density, these batteries are frequently used in devices like hearing aids.
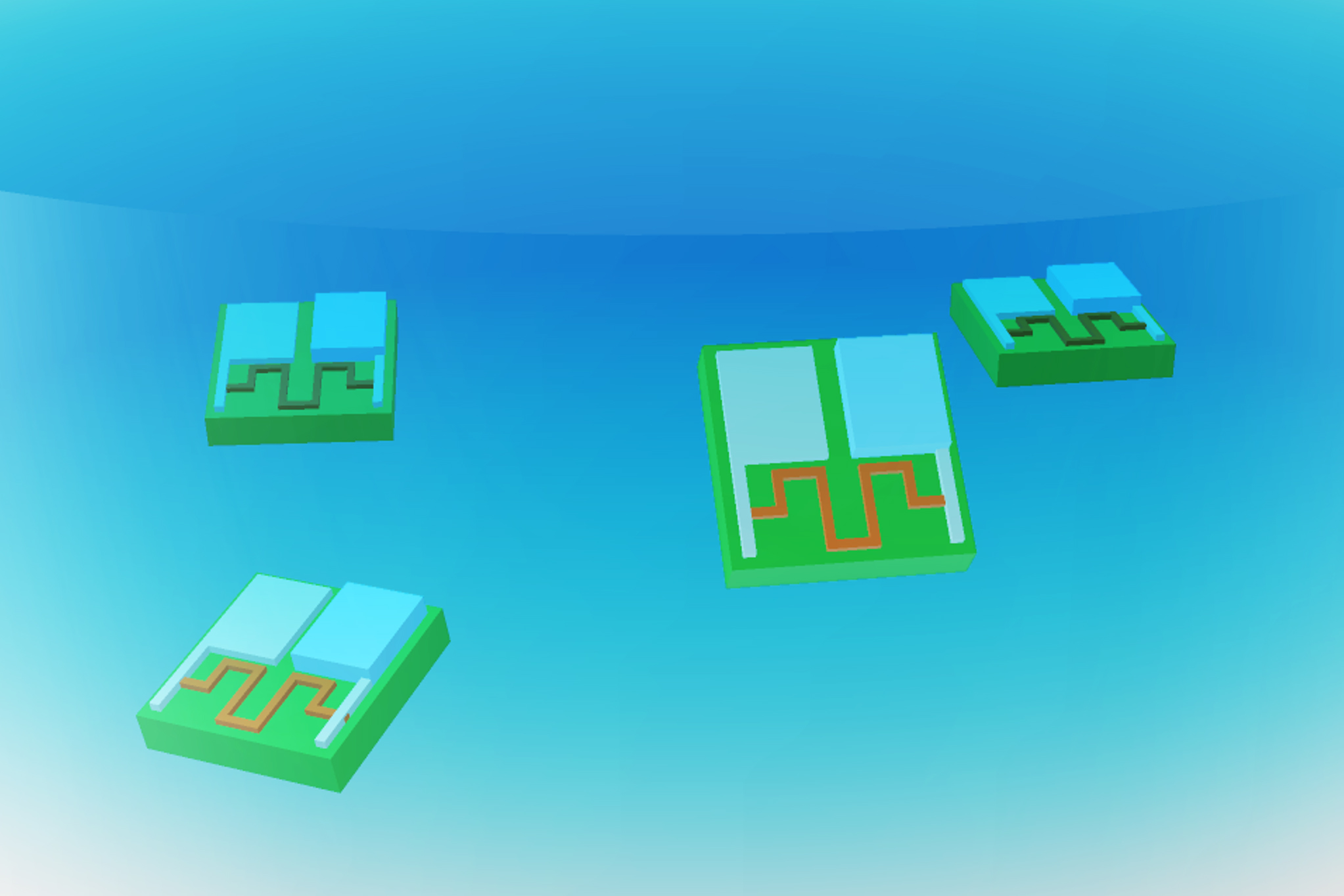
The battery developed by the researchers features a zinc electrode paired with a platinum electrode, both embedded within a polymer strip called SU-8, commonly used in microelectronics. When oxygen molecules from the air interact with these electrodes, zinc gets oxidized, releasing electrons that flow to the platinum electrode, thus generating electric current.
In their experiments, the researchers demonstrated that this battery can efficiently power an actuator—in this instance, a robotic arm capable of movement. It can also energize a memristor that stores memories based on changes in electrical resistance, as well as a clock circuit, helping robotic devices keep time.
The battery’s output can also support two types of sensors that adjust their electrical resistance in response to different chemicals in the environment, one constructed from atomically thin molybdenum disulfide and the other from carbon nanotubes.
“We are creating foundational building blocks to develop functionalities at the cellular level,” notes Strano.
Building the Future of Robotics
In the study, the researchers connected their battery to an external device via a wire. However, future projects aim to fully integrate the battery directly into the robots themselves.
“This battery will serve as a central component for many of our robotics initiatives,” Strano states. “Just as an electric car is designed around its battery, we can develop robots centered on an energy source.”
One key area of exploration involves crafting tiny robots that could be injected into the human body to target specific sites for drug delivery, such as administering insulin. These devices would utilize biocompatible materials designed to dissolve once their purpose is fulfilled.
The team is also working on enhancing the battery’s voltage, potentially expanding its applications even further.
This innovative research received funding from the U.S. Army Research Office, the U.S. Department of Energy, the National Science Foundation, and a MathWorks Engineering Fellowship.
Photo credit & article inspired by: Massachusetts Institute of Technology