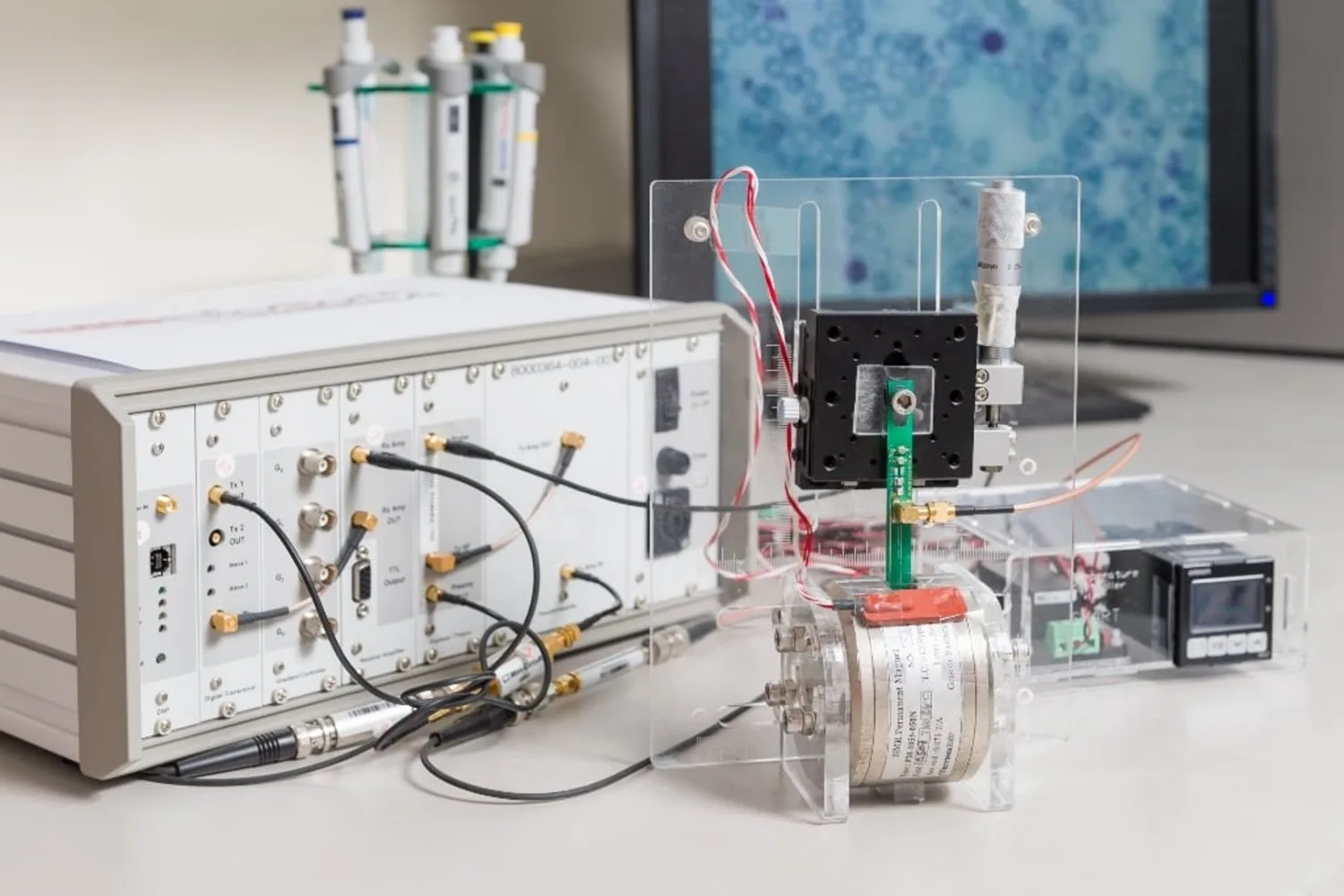
For individuals dealing with paralysis or limb loss, neuroprosthetics that utilize electrical stimulation to trigger muscle contractions can restore some level of mobility. Despite extensive research, these electrically-controlled prostheses remain underutilized due to issues like rapid muscle fatigue and lack of precise control.
Researchers at MIT are pioneering a groundbreaking approach that could radically enhance muscle control while minimizing fatigue. Instead of relying on electrical impulses, they have turned to light. In their research conducted on mice, they demonstrated how this innovative optogenetic method not only allows for superior muscle control but also significantly reduces fatigue.
“Utilizing light through optogenetics enables more natural muscle control. This type of interface holds immense potential for clinical applications,” explains Hugh Herr, a professor at MIT and co-director of the K. Lisa Yang Center for Bionics.
Optogenetics involves genetically modifying cells to express light-sensitive proteins, granting researchers the ability to control these cells’ behavior with light exposure. While this method is not yet practical for use in humans, Herr and MIT graduate student Guillermo Herrera-Arcos, along with their colleagues at the K. Lisa Yang Center for Bionics, are exploring safe and effective ways to introduce these proteins into human tissues.
Herr serves as the senior author of a study published today in Science Robotics, with Herrera-Arcos as the lead author.
Understanding Optogenetic Control
For years, scientists have investigated functional electrical stimulation (FES) to manage muscle movement. This technique involves implanting electrodes that stimulate nerves, causing muscle contractions. However, FES often activates entire muscles simultaneously, contrasting with the body’s natural and refined method of muscle control.
“Humans naturally recruit muscle fibers in a precise order—from small to large motor units, based on the strength of the neurological signal,” Herr explains. “In FES, the largest motor units are engaged first, resulting in a sudden burst of force, which complicates fine muscle control and accelerates fatigue.”
In this study, the MIT team aimed to revolutionize the interface used in muscle stimulation. Instead of traditional electrodes, they opted to manage contractions using light-driven molecular machines via optogenetics.
Working with mice genetically engineered to produce the light-sensitive protein channelrhodopsin-2, the researchers implanted a tiny light source near the tibial nerve, which triggers the muscles in the lower leg.
By gradually increasing the intensity of the light, they observed a steady and proportional muscle contraction, unlike the sudden and inconsistent response seen with FES. “The optical stimulation allows us to control muscle force in a smooth, linear manner, similar to how our brain naturally commands muscle activity,” Herrera-Arcos notes.
Enhanced Fatigue Resistance
From their experimental findings, the researchers developed a mathematical model of optogenetic muscle control that correlates the quantity of light received with the muscle’s output force.
This model enabled them to engineer a closed-loop controller that sends signals to stimulate the muscle. After contraction, a sensor measures the exerted force, relaying this information back to adjust the light stimulation as needed for the desired muscle output.
Intriguingly, muscles could be stimulated for more than an hour without showing signs of fatigue through this method, compared to a mere 15 minutes with FES stimulation.
A challenge that remains is ensuring the safe delivery of light-sensitive proteins into human tissue. Previous research from Herr’s lab revealed that such proteins could provoke an immune reaction in rats, potentially leading to muscle degeneration and cell death.
“A major focus of our center is addressing this challenge,” Herr emphasizes. “We are pursuing multiple strategies to create innovative light-sensitive proteins that can be delivered without triggering an immune response.”
In addition to this, Herr’s team is developing new muscle force and length sensors, as well as exploring implantable light sources. If successful, these advancements could significantly benefit individuals recovering from strokes, limb amputations, spinal cord injuries, and other conditions that impair limb control.
“Ultimately, our work could facilitate a minimally invasive solution, transforming clinical care for those suffering from various limb-related disorders,” Herr concludes.
This research has been funded by the K. Lisa Yang Center for Bionics at MIT.
Photo credit & article inspired by: Massachusetts Institute of Technology