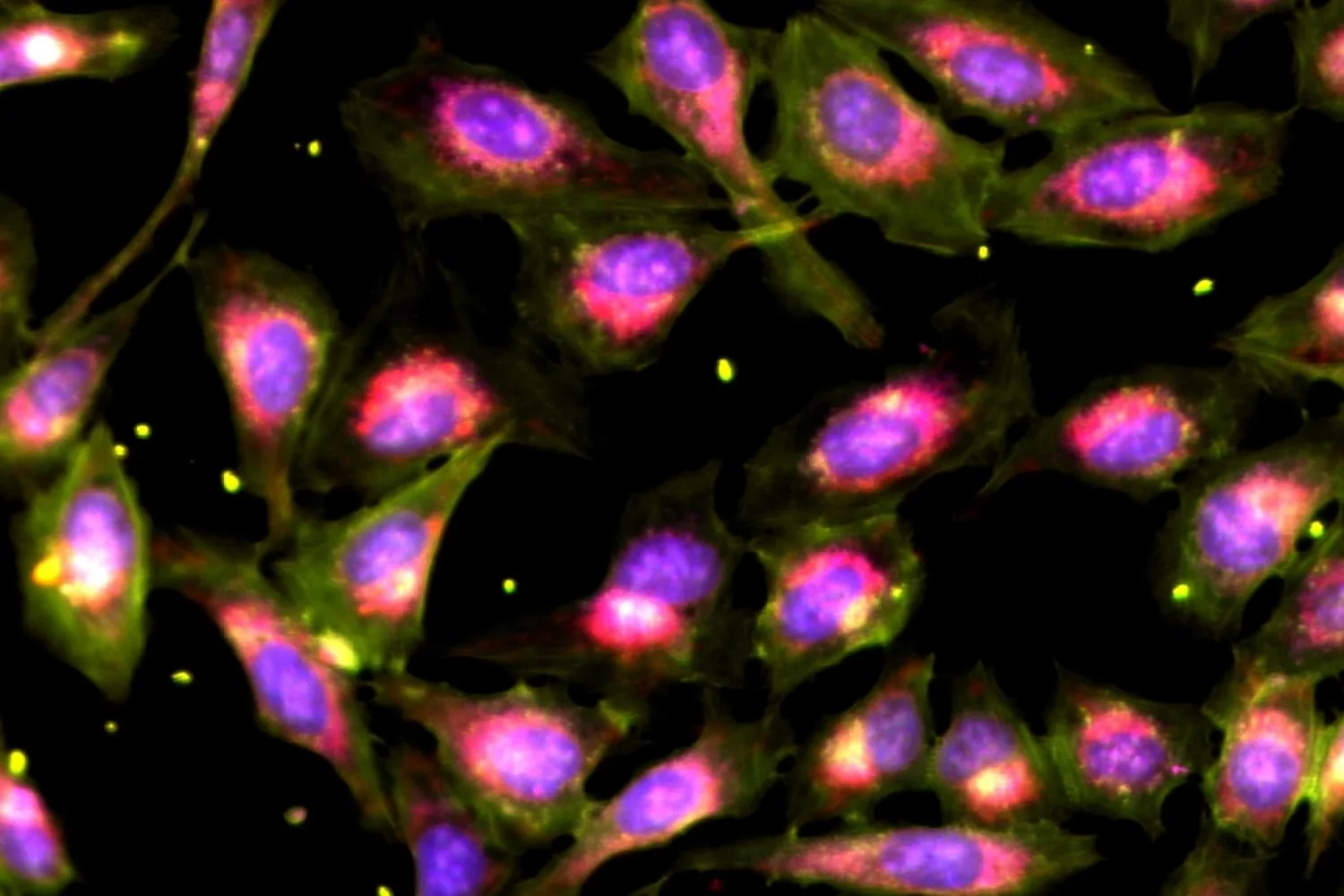
Innovative breakthroughs in drug discovery often stem from a method known as **phenotypic screening**, which has played a pivotal role in unveiling some of the world’s most widely used medications, such as penicillin. This approach involves applying various drugs to a biological challenge—like inhibiting bacterial growth or rectifying cellular defects—and closely observing the outcomes, often without an initial understanding of how the drugs interact with the biological targets. Surprisingly, historical evidence indicates that such broad-spectrum investigations have a higher success rate in leading to approved therapies than those concentrating solely on specific molecular targets.
A key factor contributing to this success is how effectively scientists frame the problem at hand. For instance, certain microbial infections or single genetic mutations are relatively straightforward to model, whereas complex diseases like cancer require multifaceted biological models that are challenging to create and evaluate. Thus, researchers face significant hurdles in testing a wide array of potential drug candidates, which limits the full advantages of phenotypic screening.
Now, researchers from the Shalek Lab at MIT have introduced an innovative technique that enhances the scalability of phenotypic screening. This novel approach enables scientists to administer multiple drugs simultaneously to a biological issue and then decipher the individual effects using computational analysis. When applied to models of pancreatic cancer and human immune cells, this method yielded unexpected biological insights while substantially reducing costs and sample requirements—addressing multiple research challenges at once.
“This method demonstrates incredible potential,” says Zev Gartner, a pharmaceutical chemistry professor at the University of California, San Francisco. “If a strong phenotype is targeted, this could be a powerful approach.”
The findings were published on October 8 in Nature Biotechnology, led by Ivy Liu and her colleagues, including Walaa Kattan, Benjamin Mead, and Conner Kummerlowe, as well as Alex K. Shalek, the director of MIT’s Institute for Medical Engineering and Sciences (IMES). The project received backing from the National Institutes of Health and the Bill and Melinda Gates Foundation.
A “crazy” way to scale up
Advancements in technology over the last decade have fundamentally transformed our comprehension of single-cell dynamics, paving the way for richer phenotypic screens. Yet, challenges persist.
For instance, biologically relevant models such as organoids and primary tissues are often scarce, while highly informative procedures like single-cell RNA sequencing can be costly and labor-intensive.
This predicament prompted the team to explore a “bold, possibly crazy idea,” according to Liu, a PhD candidate in the MIT Computational and Systems Biology program. They opted to merge numerous perturbations—drugs, chemical agents, or biologically derived compounds—into a single mixture and subsequently analyze the effects of each component.
The team began by testing various combinations of 316 U.S. Food and Drug Administration-approved drugs. Liu describes it as setting a “high bar,” with the assumption that disentangling the signals from these established drugs could be exceptionally challenging.
These diverse mixtures included between three and 80 drugs per combination, tested on lab-cultivated cells. The team applied a linear computational model to interpret the individual drug effects within each pool.
The results were promising; when compared with traditional methods of testing individual drugs, this new approach produced similarly accurate findings while dramatically cutting costs, required samples, and effort.
Real-world applications
To examine the method’s real-world relevance, the researchers tackled two significant health challenges that were previously deemed unapproachable using conventional phenotypic strategies.
The first inquiry focused on pancreatic ductal adenocarcinoma (PDAC), one of the most lethal cancer types. In PDAC, a myriad of signals emanate from surrounding tumor cells, influencing tumor progression and treatment responses. The researchers aimed to pinpoint the most significant signals affecting these dynamics.
By leveraging their novel method, they pooled various biological signals and unexpectedly identified several critical candidates. According to Shalek, “Some of our discoveries were completely unforeseen,” which included two overlooked cytokines that could predict patient survival outcomes based on public cancer datasets.
The second investigation assessed how 90 different drugs could modulate immune system functions. These agents were tested on fresh human blood cells, rich in various immune cell types. Implementing their new methodology alongside single-cell RNA sequencing allowed the team to not only explore an extensive drug library but also to delineate the individual effects on each cell type. This approach enabled a more nuanced understanding of how drugs perform in complex tissues, assisting in identifying the most effective therapies.
Shalek emphasizes, “We might target a drug to correct a defect in a T cell without considering its effects on other surrounding cells.” This method now offers a pathway to collect comprehensive data to optimize drug selection, ensuring maximum efficacy while minimizing potential side effects.
These experiments underscore the necessity for improved tools and datasets to formulate better treatment hypotheses. Shalek concludes, “The complexity and unpredictability of our findings suggest we are likely overlooking the most effective drugs in many cases.”
Streamlining drug discovery
While the innovative compression technique does excel in identifying the most impactful perturbations, it cannot entirely isolate each one’s effects. Therefore, the team suggests that this method should supplement traditional screening processes. “Follow-up traditional tests on the top hits are essential,” advises Liu.
Crucially, this new framework significantly reduces the input samples, costs, and labor involved in conducting screens. By lowering these obstacles, it represents an exciting breakthrough in understanding complex cellular responses and developing new precision medicine strategies.
Shalek remarks, “This approach opens up vast possibilities for identifying the right targets and therapies that can enhance patient outcomes.”
Photo credit & article inspired by: Massachusetts Institute of Technology