The human genome comprises approximately 23,000 genes, yet only a small percentage are active within any given cell at a given moment. Central to the intricate mechanisms that regulate gene expression are elements known as enhancers. These enhancers can often be situated far from the genes they influence, which complicates our understanding of their functional relationships.
To tackle this complexity, MIT researchers have developed an innovative technique that highlights the timing of gene and enhancer activation within a cell. When a gene is activated concurrently with a specific enhancer, it strongly indicates that the enhancer is instrumental in regulating that gene.
Gaining insights into which enhancers govern particular genes across diverse cell types could lead to identifying potential drug targets for genetic disorders. Numerous genomic studies have linked mutations in non-protein-coding regions to a variety of diseases, raising the question: could these be undiscovered enhancers?
“Many of the regions of chromosomes identified through genetic technology as having disease implications do not correspond to known genes. We suspect they point to enhancers that might be situated far from gene promoters, making it crucial to accurately identify these enhancers,” states Phillip Sharp, an MIT Institute Professor Emeritus and member of MIT’s Koch Institute for Integrative Cancer Research.
Sharp serves as the senior author of the recent study published today in Nature. The lead author of the paper is MIT Research Assistant D.B. Jay Mahat.
Searching for Enhancer RNA (eRNA)
Less than 2% of the human genome consists of protein-coding genes, while the majority contains elements that regulate how and when these genes are expressed. Enhancers, identified around 45 years ago, are believed to activate genes through physical contact with gene promoter regions via complex formations.
In 2010, researchers discovered that these enhancers transcribe into RNA molecules, which are termed enhancer RNA or eRNA. This transcription likely occurs during the enhancers’ active interaction with their target genes. This discovery prompted the idea that measuring eRNA transcription levels could help determine enhancer activity and the genes they target.
“Understanding when enhancers are active is vital for grasping developmental processes and the regulatory changes associated with cancer progression,” says Mahat.
However, mapping eRNA has proven challenging due to its small production levels and short-lived presence in cells. Additionally, eRNA is characterized by the absence of a poly-A tail, a modification that most existing techniques rely on to extract RNA from cells.
One effective approach to capture eRNA involves incorporating a specialized nucleotide into cells that halts transcription upon its integration into RNA, allowing the RNA to be tagged with a biotin marker for extraction. Nonetheless, this method typically requires large cell pools and lacks individual cell resolution.
During their exploration of new eRNA capture methods, Mahat and Sharp contemplated the application of click chemistry, a process that enables the joining of two molecules by using “click handles” that react together.
The researchers fashioned nucleotides tagged with one click handle. Once integrated into elongating eRNA strands, these strands could be retrieved using complementary handles. This innovative method enabled the researchers to successfully capture, purify, amplify, and sequence eRNA—estimating they could recover around 10% of the eRNA from a single cell.
This technique allowed the scientists to obtain a real-time snapshot of the enhancers and genes actively transcribed in a cell.
“Our goal is to identify, within individual cells, how transcription from regulatory elements corresponds with their associated genes. This level of analysis is crucial for detecting the synchronous or asynchronous activation between genes and their regulatory elements,” Mahat explains.
Analyzing Gene Expression Timelines
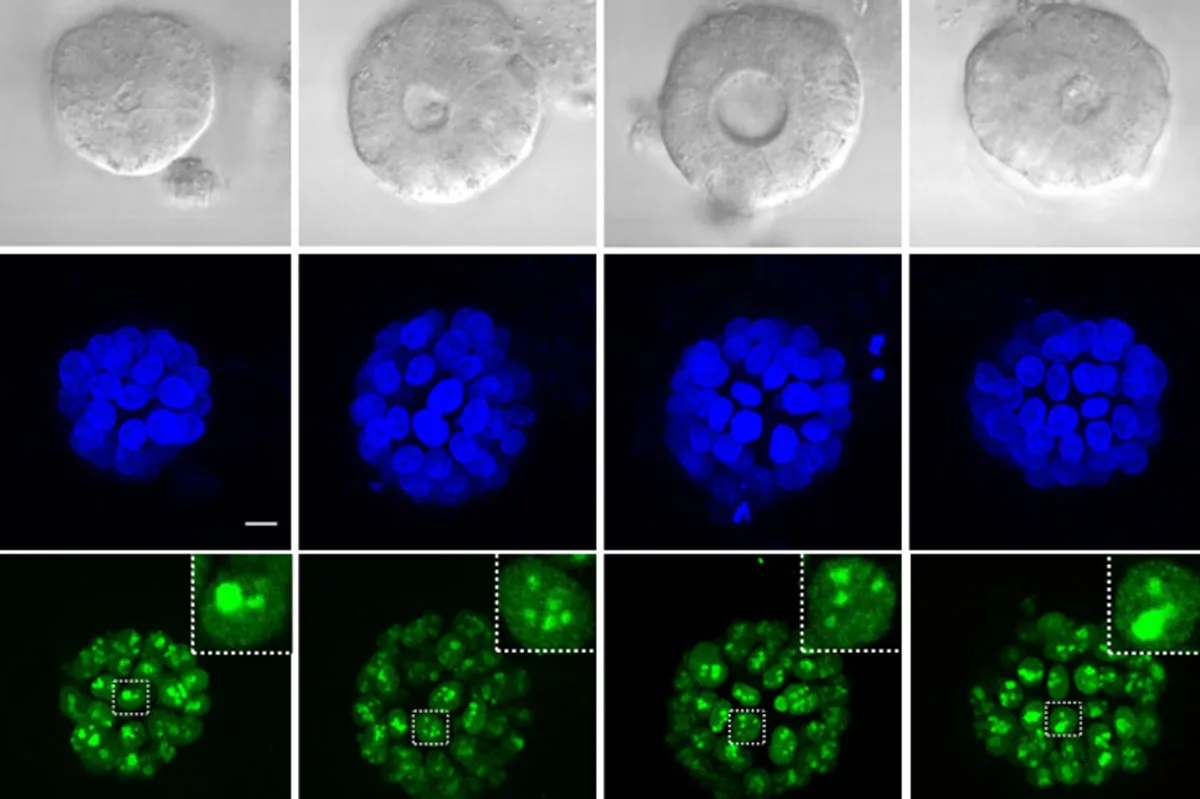
Demonstrating the technique using mouse embryonic stem cells, the researchers successfully estimated when particular regions began to transcribe by examining the RNA strand lengths and the transcription speed of polymerase, which facilitates this process. This allowed them to identify which genes and enhancers were transcribing concurrently.
The research team honed in on the timing of cell cycle gene expression more precisely than ever before. They verified several known gene-enhancer pairs and compiled a substantial list of around 50,000 potential enhancer-gene associations for further validation.
Understanding the enhancer-gene relationships is pivotal for crafting new treatments for genetic disorders. For instance, the FDA recently approved the first gene therapy for sickle cell anemia, which operates by interfering with an enhancer that activates a fetal globin gene, consequently reducing the sickling of blood cells.
Currently, the MIT team is extending this analysis to other cell types, with a specific emphasis on autoimmune diseases. They are collaborating with researchers from Boston Children’s Hospital to investigate immune cell mutations linked to lupus, many of which occur in non-coding genomic regions.
“The impact of these mutations on gene function remains unclear, prompting us to begin unraveling the genes that these likely enhancers may regulate and in which cell types they are most active,” Mahat highlights. “This serves as a foundational tool for generating gene-to-enhancer maps, critical for biological understanding and disease research.”
Furthermore, the findings support a theory proposed by Sharp, alongside MIT professors Richard Young and Arup Chakraborty—that transcription is regulated by membraneless droplets known as condensates. These condensates comprise extensive clusters of enzymes and RNA, possibly including eRNA generated at enhancer sites.
“We envision that the interaction between an enhancer and a promoter occurs within a transient condensate structure, where RNA plays a vital role,” Sharp concludes.
The research received funding from the National Cancer Institute, the National Institutes of Health, and the Emerald Foundation Postdoctoral Transition Award.
Photo credit & article inspired by: Massachusetts Institute of Technology