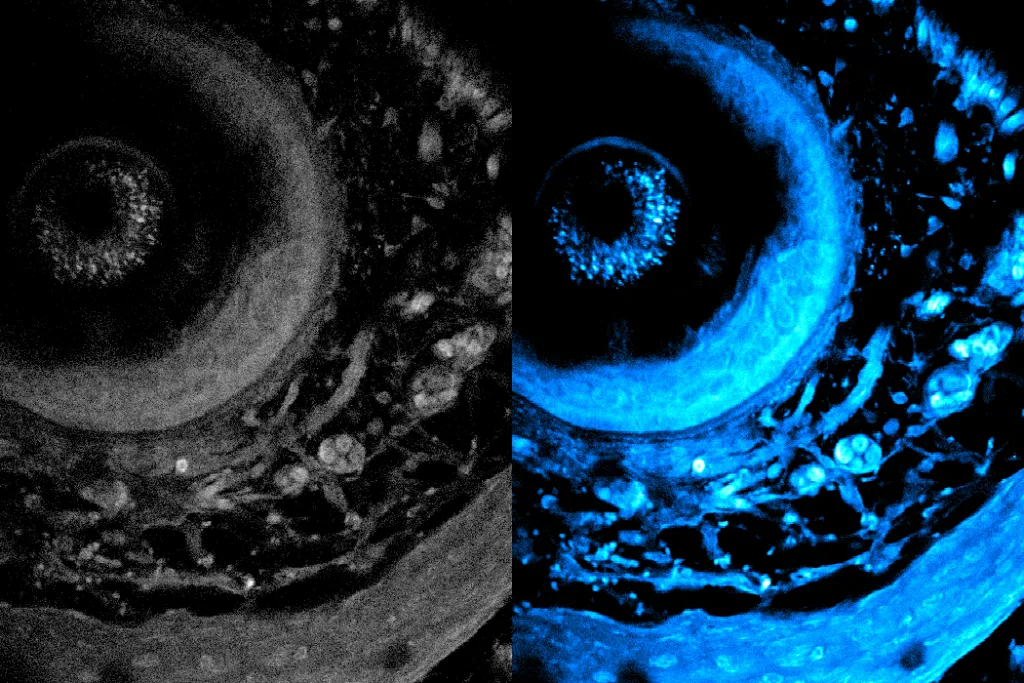
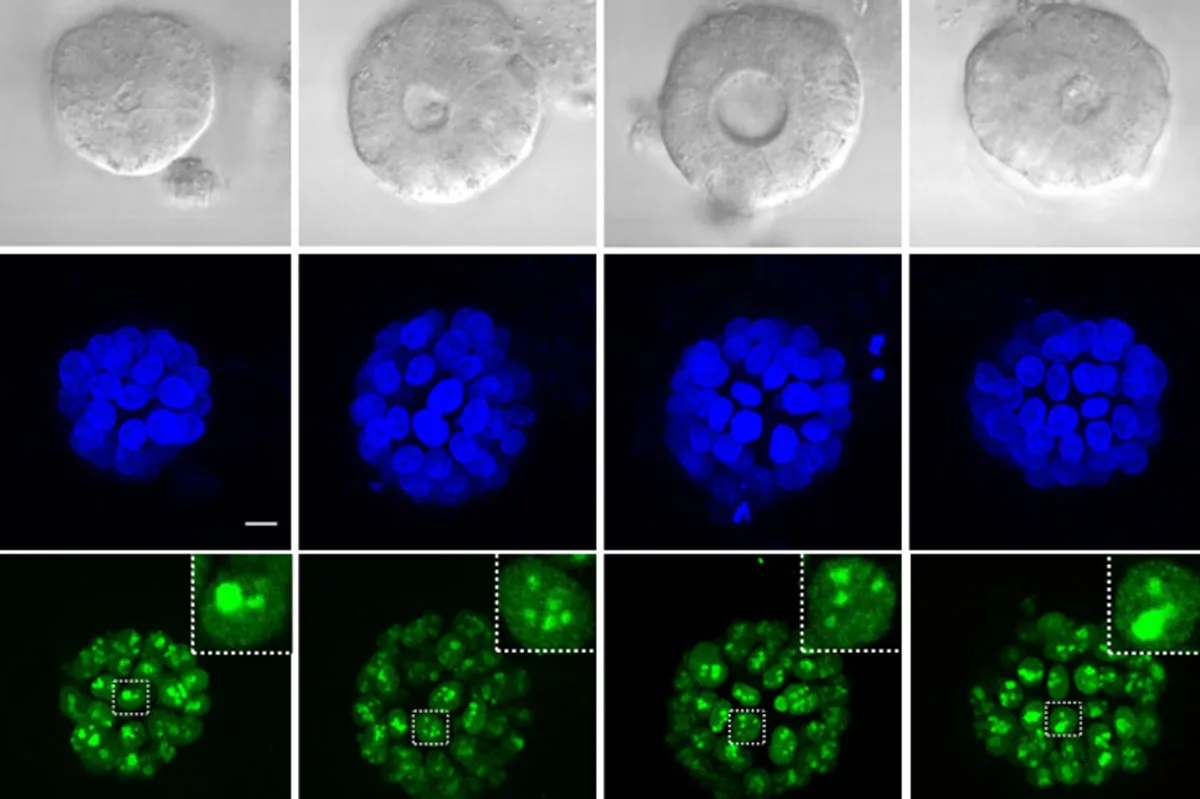
Metabolic imaging is an innovative, noninvasive technique that allows researchers and medical professionals to examine living cells with the use of laser light. This cutting-edge method aids in evaluating disease progression and treatment responses effectively.
However, one of the primary challenges with light-based imaging is its limited penetration into biological tissues due to scattering, which compromises the resolution of the obtained images.
Recently, researchers at MIT have unveiled a groundbreaking technique that significantly enhances the depth limit of metabolic imaging, more than doubling it. This advancement also accelerates imaging speeds, resulting in richer and more detailed images.
What sets this new method apart is that it does not require any preprocessing of tissue samples, such as slicing or staining. Instead, it utilizes a specialized laser to penetrate deeper into tissue, prompting intrinsic molecules within cells to emit light. This approach preserves the natural integrity of the tissue, enabling a more accurate representation of its structure and function.
The team achieved this breakthrough by adaptively customizing the laser light for deep tissue imaging. By employing a versatile fiber shaper—an adaptable device that can be manipulated for optimal light delivery—they fine-tune the colors and pulses of the laser to minimize scattering and enhance the signal as it penetrates deeper into the tissue. This innovation allows for unprecedented clarity in imaging living tissues.
Credit: Courtesy of the researchers
With greater depth penetration, faster imaging speeds, and higher resolution, this technique is particularly advantageous for complex applications such as cancer research, tissue engineering, drug discovery, and the analysis of immune responses.
Sixian You, an assistant professor in the Department of Electrical Engineering and Computer Science (EECS) at MIT, highlights the significance of this advancement, stating, “This work demonstrates a remarkable enhancement in depth penetration for label-free metabolic imaging, opening new pathways for exploring metabolic dynamics within living biosystems.”
Joining You as co-authors of the research paper are Kunzan Liu, an EECS graduate student; Tong Qiu, a postdoctoral researcher; Honghao Cao, an EECS graduate student; and several other MIT colleagues. The findings are published in Science Advances.
The Innovation Behind the Method
This innovative technique is categorized as label-free imaging, which means samples are not stained prior to the imaging process. While staining enhances visibility of cellular structures such as cell nuclei and proteins, it often requires sectioning and slicing, which can compromise the viability of the tissue and hinder the observation of dynamic processes in living cells.
Label-free imaging leverages lasers to excite specific molecules in cells, causing them to emit light of varying colors that can reveal molecular compositions and cellular architectures. However, producing optimal laser light with the right wavelengths for deep tissue imaging has posed challenges.
The MIT team has crafted a novel approach to surpass this limitation. Utilizing a multimode optical fiber—capable of handling substantial power—paired with a compact fiber shaper, they can meticulously control light propagation by adjusting the fiber’s shape. This alteration modifies the laser’s color and intensity, enabling deeper imaging capabilities.
“We aim to direct all this energy into the precise wavelengths and pulse characteristics necessary for efficient imaging. This results in clearer images, even at considerable depths within tissues,” says Honghao Cao.
Once the adjustable fiber mechanism was developed, an imaging platform was constructed to exploit the robust laser source, generating longer wavelengths crucial for penetrating biological tissues more effectively.
“We believe this technology can make significant contributions to biological research. Our goal is to make it affordable and widely accessible, empowering scientists with a transformative tool for exploration,” Liu adds.
Expanding Applications
In practical applications, the new imaging system has demonstrated the ability to penetrate over 700 micrometers into biological samples, dwarfing the previous limit of around 200 micrometers.
“This innovative deep imaging technique allows us to explore biological samples in ways never before possible,” Liu states.
With the ability to visualize cells at various depths within living systems, researchers can now investigate metabolic alterations that occur throughout different layers of tissue. Additionally, the enhanced speed of imaging facilitates the collection of extensive data regarding the interplay between cellular metabolism and mobility.
This advanced imaging method holds significant promise for studying organoids—engineered cellular structures designed to replicate organ functions. Researchers in the Kamm and Griffith labs are at the forefront of advancing brain and endometrial organoid development for disease research and treatment evaluation.
Traditionally, accurately observing internal processes without damaging the tissue has been challenging. The new method allows researchers to noninvasively monitor metabolic states within living organoids as they mature.
The research team aims to capture even higher-resolution images in the future. They are also working on creating low-noise laser sources for deeper imaging with reduced light dosage. Meanwhile, algorithms are being developed to reconstruct comprehensive 3D biological structures at high resolution.
Ultimately, they hope to apply this innovative technique in real-world scenarios, allowing biologists to monitor drug responses in real-time, facilitating the advancement of new therapeutic approaches.
“By enabling multimodal metabolic imaging that penetrates deeper into tissues, we provide scientists with an exceptional capability to observe nontransparent biological systems in their natural state. We are eager to collaborate with clinicians, biologists, and bioengineers to push the frontiers of this technology, ultimately transforming insights into practical medical breakthroughs,” You assures.
Experts in the field have expressed excitement over these developments. Melissa Skala from the Morgridge Institute for Research, unaffiliated with this project, comments, “This work is thrilling as it employs innovative feedback methods to enable deeper imaging of cell metabolism compared to existing techniques. Fast imaging speeds also allow researchers to delve into unique metabolic dynamics of immune cell movement within blood vessels.”
Moreover, Irene Georgakoudi, a biomedical engineering professor at Tufts University, remarks, “Achieving high-resolution multi-photon images that leverage NAD(P)H autofluorescence contrast is pivotal for studying various significant issues in living tissues. Rapid and deep imaging is essential for ensuring data relevance, sampling meaningful tissue volumes, and tracking rapid changes.”
This research is partially funded by MIT startup initiatives, the U.S. National Science Foundation CAREER Award, an MIT Irwin Jacobs and Joan Klein Presidential Fellowship, and an MIT Kailath Fellowship.
Photo credit & article inspired by: Massachusetts Institute of Technology