In today’s world, most electronic devices operate through the movement of electrons. However, researchers are discovering that materials capable of efficiently conducting protons—the nucleus of hydrogen atoms—could play a pivotal role in several technologies aimed at tackling climate change.
Currently available inorganic materials that conduct protons typically require high temperatures to function effectively. This limitation hinders the development of advanced technologies such as more efficient fuel cells for generating clean electricity from hydrogen, electrolyzers for producing clean fuels like hydrogen for vehicles, solid-state proton batteries, and innovative computing devices leveraging iono-electronic effects.
To propel the advancement of proton conductors, engineers from MIT have pinpointed specific characteristics in materials that lead to rapid proton conduction. By quantifying these traits, the team identified several new candidates that show significant promise as efficient proton conductors. Simulations indicate that these materials could outperform existing options, although experimental validation is still needed. This research not only uncovers potential new materials but also enhances our understanding of the atomic mechanisms behind their functionality.
The recent findings are detailed in a paper published in the journal Energy and Environmental Sciences, authored by MIT professors Bilge Yildiz and Ju Li, alongside postdoctoral researchers Pjotrs Zguns and Konstantin Klyukin, and collaborator Sossina Haile from Northwestern University. Yildiz holds the Breene M. Kerr Professorship in Nuclear Science and Engineering and Materials Science and Engineering.
“Proton conductors are essential in clean energy conversion applications like fuel cells, which utilize hydrogen to generate electricity without carbon dioxide emissions,” explains Yildiz. “Our goal is to enhance this process’s efficiency by utilizing materials that can transfer protons rapidly through these devices.”
Conventional hydrogen production methods, such as steam methane reforming, release a substantial amount of carbon dioxide. “Electrochemically producing hydrogen from water vapor is one effective solution, but it demands high-quality proton conductors,” Yildiz notes. This efficiency is also critical for the production of various industrial chemicals and fuels, including ammonia.
Most inorganic proton-conducting materials currently available operate only at temperatures between 200 and 600 degrees Celsius (approximately 450 to 1,100 degrees Fahrenheit) or even higher. Maintaining these elevated temperatures consumes energy and can degrade materials over time. Yildiz adds, “Higher temperatures can complicate systems and impact material durability. As of now, there are no viable inorganic proton conductors at room temperature.” The sole known room-temperature proton conductor is a polymer that lacks scalability for computing applications.
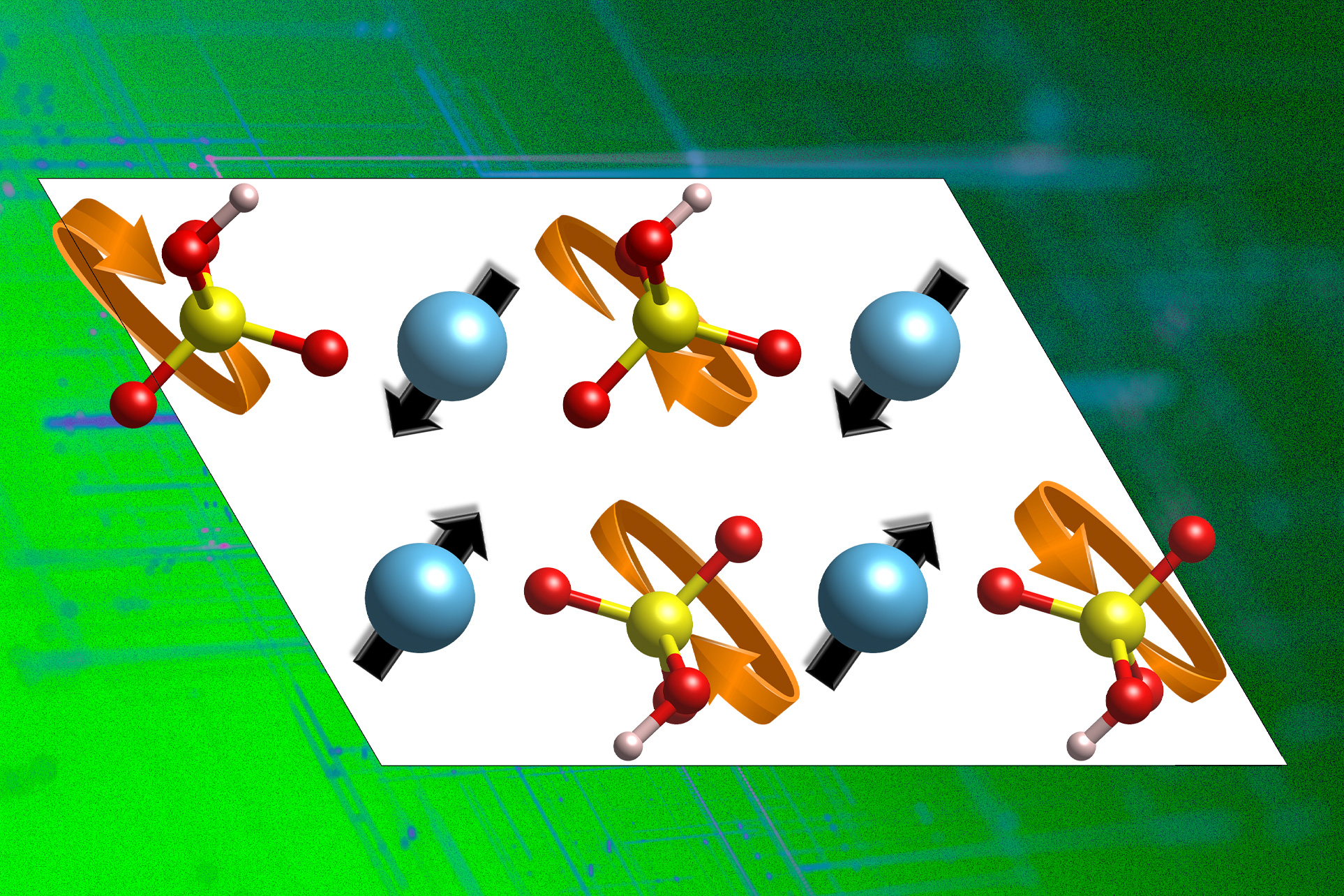
Addressing these challenges, the research team developed a fundamental understanding of how proton conduction operates in inorganic compounds, specifically solid acids. “To advance, we needed to grasp the governing principles of proton conduction in these materials,” Yildiz affirms. By studying the atomic arrangements, they identified critical characteristics influencing proton-carrying capabilities.
Yildiz elaborates that proton conduction involves a proton “hopping” from a donor oxygen atom to an acceptor one, followed by a reorganization of the surrounding environment to facilitate the proton’s movement to neighboring acceptors, enabling long-range diffusion. “Understanding the structural reorganization necessary for this process was a key insight in our research,” she states.
Utilizing computer simulations, the researchers explored solid acids—materials that exhibit strong proton conduction at elevated temperatures. These materials feature a substructure known as the polyanion group sublattice, which must rotate to displace the proton from its initial position for effective transfer. The research team identified the phonons that enhance the sublattice’s flexibility, which is vital for proton conduction. With this knowledge, they sifted through extensive databases of potential compounds to find better proton conductors.
The outcome of their efforts was the discovery of solid acid compounds with promising proton conduction properties that had previously been overlooked in this context. These compounds exhibited the ideal lattice flexibility needed for effective conduction. The team then performed simulations to assess how these identified materials would perform at relevant temperatures, confirming their suitability for applications in fuel cells and beyond. Encouragingly, they identified six materials predicted to conduct protons faster than the best-known solid acid options.
While Yildiz acknowledges some uncertainties in these simulations, she is optimistic. “Although I can’t precisely quantify the conductivity improvements, these materials are highly promising. We hope this spurs experimental efforts to synthesize and explore these compounds as practical proton conductors.”
The transition from these theoretical discoveries to real-world applications may take some time, but Yildiz anticipates that the first implementations will likely be in electrochemical cells for producing fuels and chemical precursors such as hydrogen and ammonia.
This research was made possible through funding from the U.S. Department of Energy, the Wallenberg Foundation, and the U.S. National Science Foundation.
Photo credit & article inspired by: Massachusetts Institute of Technology