Researchers affiliated with the Critical Analytics for Manufacturing Personalized Medicine (CAMP) interdisciplinary research group at the Singapore-MIT Alliance for Research and Technology (SMART) have made significant strides in enhancing the capacity of mesenchymal stromal cells (MSCs) to generate cartilage tissue. By incorporating ascorbic acid during MSC expansion, they have innovated a novel method that holds promise for improving cartilage regeneration. Moreover, their study highlights micro-magnetic resonance relaxometry (µMRR) as a rapid, label-free tool for monitoring the quality of expanded MSCs.
Articular cartilage, which serves as a protective cushion at the joints, is prone to degradation due to injury, aging, or conditions like arthritis, resulting in severe joint pain and disability. This issue is particularly pressing in places like Singapore, where an active and aging population faces growing challenges linked to cartilage degeneration. Currently, the only FDA-approved cell-based therapy for cartilage injuries is autologous chondrocyte implantation, a method that is expensive, labor-intensive, and often requires multiple procedures. MSCs present a viable alternative, boasting excellent safety profiles for transplantation. However, their clinical applications are often hampered by inconsistent results tied to factors such as variability between donors and non-standardized manufacturing protocols.
The inherent heterogeneity of MSCs can lead to unpredictable biological responses and treatment outcomes. To achieve a therapeutically relevant quantity of MSCs, large-scale expansions are necessary, yet this process often contributes to increased cell heterogeneity. Thus, there is an urgent need for improved methodologies that can minimize variability while maximizing the expansion of cells with enhanced chondrogenic potential—the ability of MSCs to differentiate into cartilage cells capable of repair.
In a recent paper titled “Metabolic modulation to improve MSC expansion and therapeutic potential for articular cartilage repair”, published in the journal Stem Cell Research and Therapy, CAMP researchers introduced a priming strategy that optimizes the energy utilization of MSCs, thereby enhancing their expansion. Their findings reveal a strong connection between chondrogenic potential and oxidative phosphorylation (OXPHOS), a process in which oxygen reduction generates adenosine triphosphate—an essential energy source for cellular processes. This insight suggests that manipulating MSC metabolism could serve as a promising strategy to boost their chondrogenic capability.
Utilizing innovative process analytical tools developed by CAMP, researchers investigated the effects of metabolic modulation throughout both short- and long-term harvesting and reseeding periods. By adjusting the nutrient landscape—encompassing glucose, pyruvate, glutamine, and ascorbic acid—they were able to improve MSC chondrogenic potential. Given ascorbic acid’s known role in supporting OXPHOS and enhancing chondrogenic differentiation, the team further explored its effects during MSC expansion.
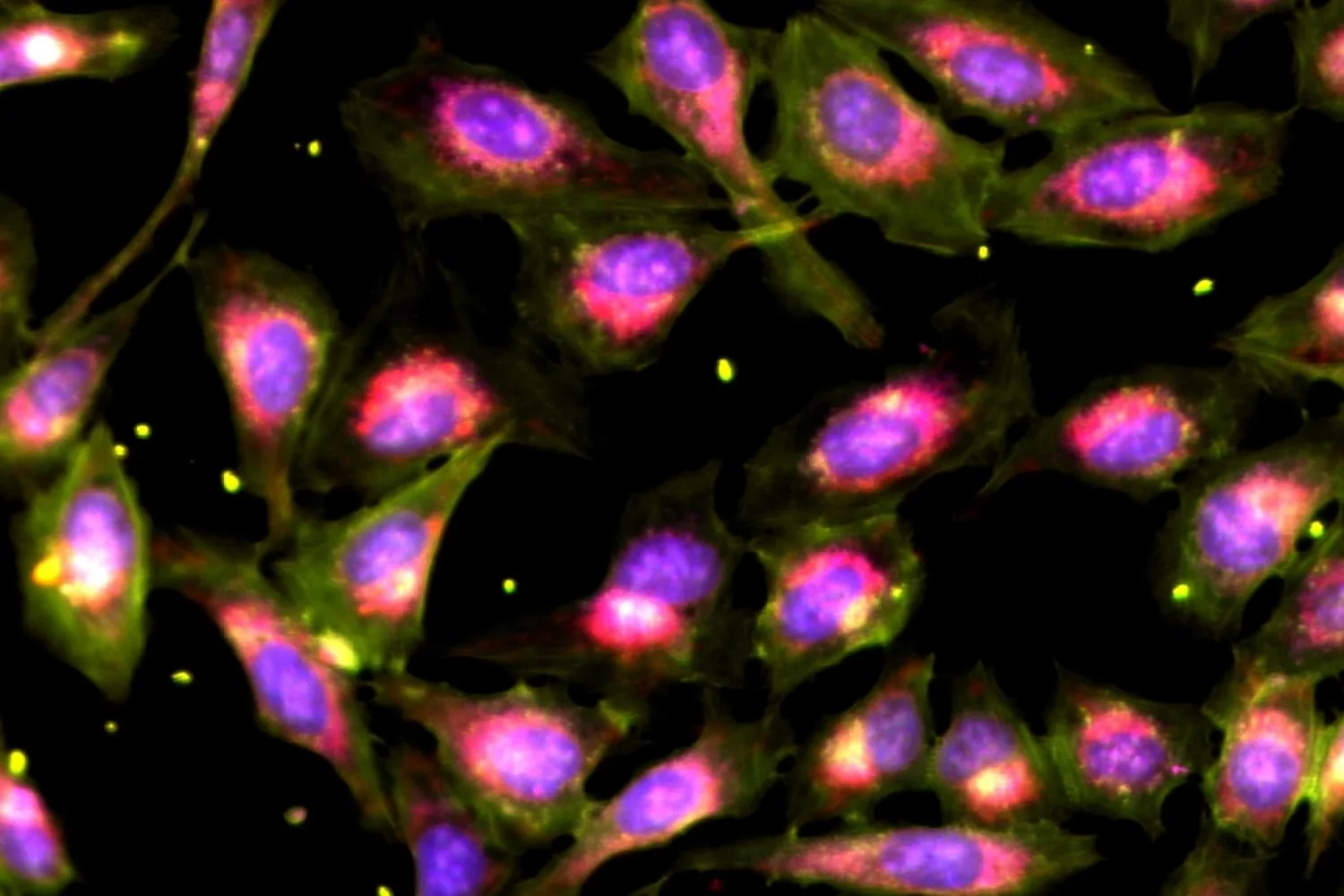
The introduction of ascorbic acid during a single passage of MSC expansion resulted in markedly improved chondrogenic differentiation, which is crucial for successful cartilage repair. Notably, prolonged ascorbic acid treatment yielded a more than 300-fold increase in MSCs with enhanced chondrogenic potential, alongside decreased heterogeneity and cellular senescence—an aging process where cells cease to divide without undergoing cell death—when set against untreated controls. Ascorbic acid-treated MSCs exhibited a pronounced metabolic shift towards OXPHOS, corroborated by μMRR measurements that identified new quality attributes that could enhance MSC manufacturing for cartilage repair.
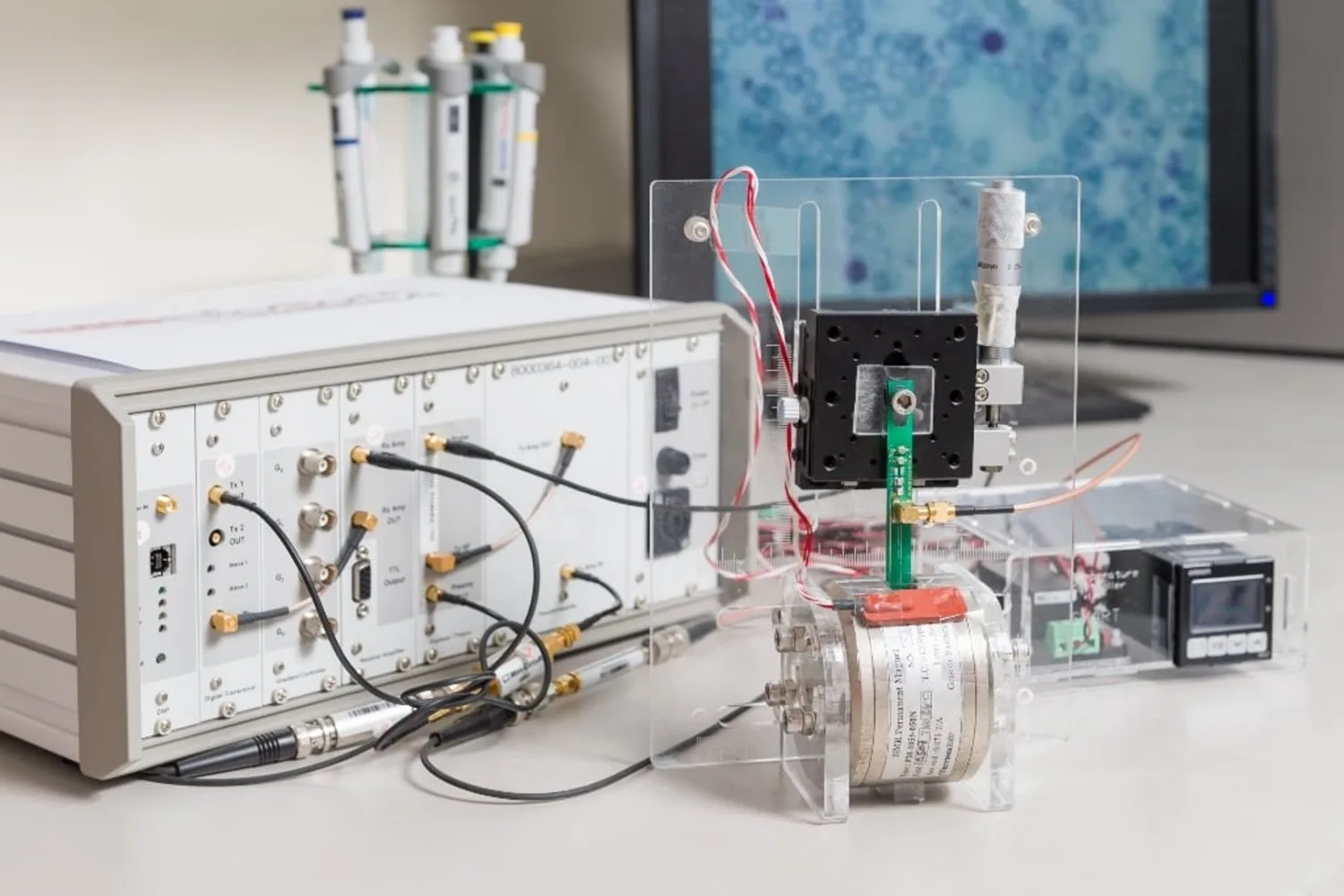
The study further underscores the efficacy of μMRR, a compact device that utilizes magnetic resonance imaging (MRI) on a micro-scale, as a monitoring tool for MSC expansion augmented by ascorbic acid. Initially employed as a label-free method for malaria detection due to paramagnetic hemozoin particles, μMRR proved valuable in assessing MSC senescence. This quick, label-free process requires minimal cell quantities, facilitating MSC therapy production in closed systems—a method designed to reduce contamination risks while allowing for periodic monitoring of smaller production lots.
“Variation among donors, heterogeneity within populations, and cellular senescence have hindered the standardization of MSCs as effective therapies for articular cartilage repair. Our work indicates that incorporating ascorbic acid during MSC expansion can mitigate these challenges and augment the chondrogenic potential of MSCs,” explains Ching Ann Tee, senior postdoctoral researcher at SMART CAMP and the paper’s lead author. “By regulating metabolic conditions like ascorbic acid supplementation alongside CAMP’s advanced analytical tools, we can significantly enhance both the yield and quality of cell therapy products. This groundbreaking approach could transform MSC therapy into a more viable treatment option and set improved standards for manufacturing protocols.”
“This metabolic modulation strategy could potentially be adapted for other therapeutic applications, such as enhancing osteogenic potential for bone repair or even in various stem cell types. Implementing our insights in MSC production settings marks a substantial advancement that could benefit patients with osteoarthritis and other joint-related disorders, as we can efficiently generate large quantities of high-quality MSCs with consistent effectiveness,” adds Professor Laurie A. Boyer, principal investigator at SMART CAMP, and corresponding author of the study.
This research effort is supported by the National Research Foundation Singapore under its Campus for Research Excellence and Technological Enterprise program.
Photo credit & article inspired by: Massachusetts Institute of Technology