Drug development is a notoriously lengthy process. It can take decades for groundbreaking research to transition into clinical trials and ultimately result in accessible medications. For individuals facing terminal illnesses, this lengthy timeline can feel unbearably distant. Sonia Vallabh, a Senior Group Leader at the Broad Institute of MIT and Harvard, knows this urgency all too well. Her research focuses on fatal familial insomnia, a type of neurodegenerative prion disease that she may inevitably face as she ages.
After discovering she carries a gene that predisposes her to this fatal condition, Vallabh and her husband, Eric Minikel, shifted their careers from unrelated fields to become researchers. Together, they lead a lab at the Broad Institute, dedicated to devising new therapies to combat prion diseases, with their own genetic timeline for success prompting a relentless pursuit of breakthroughs.
Vallabh’s collaboration with Dr. Jonathan Weissman from the Whitehead Institute has propelled their efforts considerably. In under two years, they, along with their research teams, have engineered innovative molecular tools dubbed CHARMs. These tools have the potential to silence problematic genes, such as the prion protein gene, and possibly more, thus paving the way for novel therapeutics. While significant challenges remain before their technology can be deemed clinically viable, the rapid accomplishments thus far have provided the team with renewed optimism.
“From our initial discussions, our collaboration was defined by an eagerness to expedite our research,” Vallabh mentions. “The excitement we shared propelled us into action.”
In a groundbreaking paper published in the journal Science, Weissman, Vallabh, graduate student Edwin Neumann, and postdoc Tessa Bertozzi detail their findings on CHARMs, which stands for Coupled Histone Tail for Autoinhibition Release of Methyltransferase.
“The proximity of the Whitehead and Broad Institutes fosters an ideal environment for rapid scientific advancement and innovation in medical technology,” asserts Weissman, who is also a professor of biology at MIT and an Investigator at the Howard Hughes Medical Institute. “CHARMs represent a sophisticated solution to the challenge of silencing disease genes, and they could play a crucial role in the future landscape of genetic medicine.”
Targeting Genes to Treat Genetic Diseases
Prion diseases lead to accelerated neurodegeneration and death, primarily due to abnormal versions of the prion protein. These malformed proteins trigger a chain reaction that disrupts brain function: they not only impair the normal proteins but also culminate in toxic aggregates that destroy neurons. While conditions like “mad cow disease” are infectious, others arise spontaneously or because of inherited mutations in prion protein genes.
Most traditional drugs target proteins directly, but CHARMs tackle the issue at the genetic level by silencing the gene associated with the faulty protein, preventing its formation altogether. They achieve this through a process known as epigenetic editing, which adds a chemical marker to DNA to switch off a gene without altering the DNA itself. This ensures that, unlike protein-targeting medications that require regular doses, patients may only need a single treatment with CHARMs, as the gene’s shut-off should remain stable.
Research has indicated that the prion protein isn’t essential in healthy adults, and removing it can alleviate or even resolve symptoms of the disease. This positions epigenetic editing as a promising approach for treating hereditary prion diseases, although creating an effective therapy remains a challenge.
Fortunately, the groundwork for CHARMs was laid using another tool named CRISPRoff, also produced by Weissman’s team, designed for gene silencing. CRISPRoff employs components from CRISPR gene editing technology, like Cas9, to direct the tool to the gene of interest. By adding methyl groups to the target gene, CRISPRoff effectively silences it, preventing the production of the problematic protein. Early tests confirmed CRISPRoff’s efficacy and stability in silencing the prion protein gene.
However, some characteristics of CRISPRoff made it less suitable as a therapeutic option. The researchers aimed to retain its potency while ensuring it was safe for human use, compact enough for brain delivery, and minimized the risk of off-target effects.
Transforming a Research Tool into a Drug Candidate
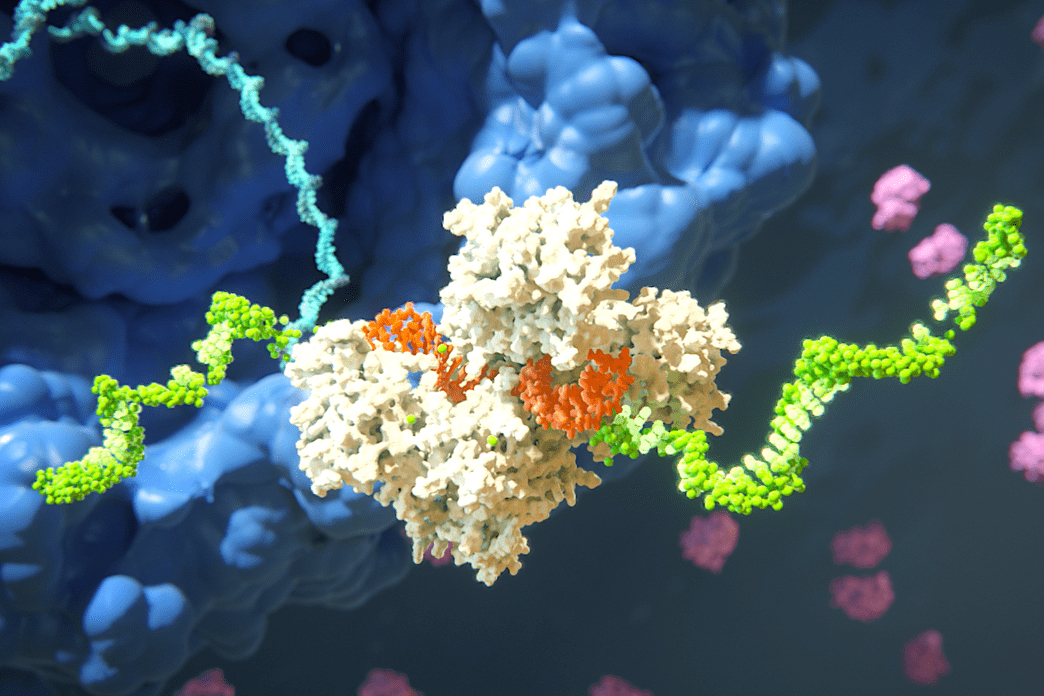
Neumann and Bertozzi spearheaded the engineering of their epigenetic editor. Their first challenge? Size. The tool must be small enough to be delivered precisely to target brain cells. Previous clinical trials have used adeno-associated viruses (AAVs) for gene delivery, but they have limitations on what they can carry. The bulky CRISPRoff’s Cas9 component took up too much space.
The research team decided to substitute Cas9 with a smaller guide protein—a zinc finger protein (ZFP). ZFPs are naturally occurring in human cells, reducing the likelihood of triggering an immune response compared to the bacterial Cas9.
The next objective was to devise a method for silencing the prion protein gene. Initially, they used a portion of DNMT3A, a methyltransferase essential for DNA methylation, but this configuration proved toxic to cells. Instead, the researchers engineered their tool to attract the cell’s own DNMT3A to the target gene, conserving space and negating toxicity risks.
Moreover, they had to find a way to activate DNMT3A, which typically requires interaction with specific partner molecules to function. Neumann cleverly combined these partner molecules’ sections with ZFPs tailored to guide them to the prion protein gene, activating DNMT3A when it arrived, hence silencing the gene.
“Leveraging the cell’s own mechanisms minimized toxicity and complexity; we’re essentially guiding cells to deactivate a gene they ordinarily leave active,” Neumann explains.
Mouse studies confirmed that the ZFP-guided CHARMs could eliminate over 80% of prion protein in the brain—significantly more than the 21% threshold known to improve symptoms.
With a powerful gene-silencing mechanism established, the team confronted concerns about off-target effects. The CHARM delivery mechanism could persist indefinitely, raising the potential for unintended side effects. Consequently, they redesigned the tool so that once it silences the prion protein gene, it subsequently shuts itself off.
Concurrently, a collaboration with Broad Institute scientist Benjamin Deverman, focusing on enhancing gene delivery throughout the brain, published recent findings in Science. While traditional AAVs have struggled to deliver genes efficiently to the adult brain—crucial for tackling whole-brain diseases like prion disorders—Deverman’s team engineered a novel AAV vector that leverages the natural iron transport pathway into the brain, bringing CHARM technology closer to clinical readiness.
Through innovative solutions, the researchers have developed a highly effective epigenetic editor that appears poised for brain delivery, showing low toxicity and minimal off-target impacts in cell cultures and animal models.
“Being part of this journey has been incredibly rewarding. Transitioning from fundamental research to potential therapies in such a brief time frame is extraordinary,” shares Bertozzi. “Our collaboration harnessed Weissman’s engineering skills, Vallabh and Minikel’s deep understanding of the disease, and Deverman’s expertise in gene delivery.”
Future Prospects
With the essential elements of the CHARM technology in place, the team is now refining the tool for enhanced efficacy, safety, and scalable production for upcoming clinical trials. They have established a modular design, allowing various components to be interchanged, paving the way for future iterations without starting from scratch. Presently, CHARMs are being evaluated in mouse models as potential treatments.
The path from basic research to clinical trials is often fraught with challenges, and the researchers recognize that CHARMs have a journey ahead before they might offer real possibilities for individuals suffering from prion diseases like Vallabh or similar genetic disorders. Nevertheless, equipped with a well-structured therapy design and promising laboratory results, the team remains optimistic, working tirelessly to bring their technology to life—not just in the distant future but as soon as possible.
Photo credit & article inspired by: Massachusetts Institute of Technology