Scientists at MIT and the Singapore-MIT Alliance for Research and Technology have developed a groundbreaking microfluidic device that could revolutionize cell therapy for patients with spinal cord injuries. This innovative technology aims to enhance the safety and effectiveness of such treatments.
In the realm of cell therapy, induced pluripotent stem cells (iPSCs) are created by reprogramming a patient’s skin or blood cells. When treating spinal cord injuries, these iPSCs must be guided to transform into progenitor cells, which have the unique ability to mature into spinal cord cells. Once prepared, these progenitor cells are transplanted back into the patient to help regenerate damaged spinal cord tissue. However, undifferentiated iPSCs pose a significant risk, as they can lead to tumor formation if not adequately addressed.
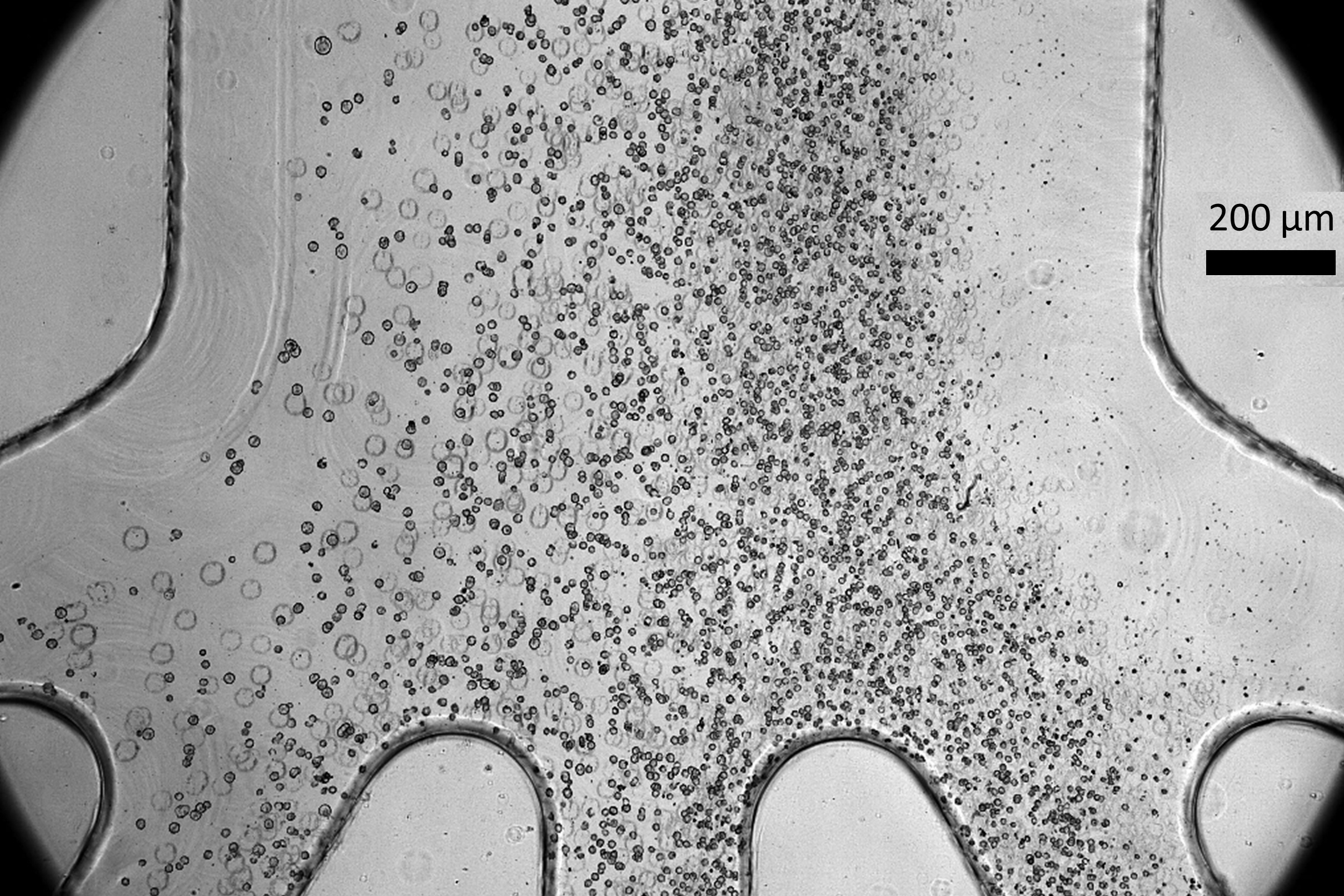
The innovative microfluidic cell sorter developed by the research team effectively eliminates approximately half of the undifferentiated cells without harming the developed progenitor cells. This high-throughput device can process over 3 million cells per minute and can be linked with additional devices to sort more than 500 million cells in a single cycle, making it a game-changer for improving cell therapy safety.
Moreover, the plastic chip housing the microfluidic cell sorter is designed for mass production at minimal costs, paving the way for broader implementation in healthcare settings. Jongyoon Han, an esteemed MIT professor and co-lead principal investigator of the CAMP (Critical Analytics for Manufacturing Personalized Medicine) research group, emphasizes the importance of cost-effective manufacturing for the widespread application of cell therapies. He states, “Our team is dedicated to enhancing the reliability and accessibility of these life-saving treatments.”
Joining Han in this pivotal research are Sing Yian Chew, a professor at Nanyang Technological University; Tan Dai Nguyen, a researcher; Wai Hon Chooi from A*STAR; and Hyungkook Jeon, an MIT postdoctoral researcher, alongside other collaborators from NTU and A*STAR. This vital research appears in the journal Stem Cells Translational Medicine.
Minimizing Cancer Risks
The presence of undifferentiated iPSCs heightens the risk of cancer development, a critical concern within cell therapy applications. Han explains that even a small number of undifferentiated cells could potentially transform into cancerous cells, making it essential for researchers to find effective methods to identify and eliminate them. While clinicians typically search for specific surface markers to distinguish these cells, no suitable markers have been identified so far. Existing chemical methods, while effective, can inadvertently damage the desired differentiated cells.
Previously, the CAMP team spent over a decade developing their high-throughput microfluidic sorter, which is capable of sorting cells based on size. Initially employed for sorting immune cells, the research team has now expanded its application to include iPSCs.
As Chew notes, “Our focus is on regenerative strategies that promote tissue repair in spinal cord injuries, which can lead to life-altering disabilities. Currently, there are limited effective regenerative treatments available.” He underscores the promise of deriving spinal cord progenitor cells from pluripotent stem cells, as they have the capability of restoring both the structure and function of spinal cord tissue. To utilize these cells effectively, ensuring their safety is paramount, which is the primary goal of their research.
Interestingly, the researchers discovered that iPSCs are generally larger than the progenitor cells derived from them. It is believed that immature pluripotent stem cells retain a multitude of genes that have yet to be suppressed during differentiation, which results in a larger nucleus. As cells mature, they significantly reduce the size of their nuclei by silencing unnecessary genes.
The microfluidic device harnesses this size disparity to sort the cells efficiently.
Innovative Spiral Sorting Technology
Within a compact plastic chip, microfluidic channels are structured to include an inlet, a spiral path, and four outlets designated for cells of varying sizes. As the cells traverse the spiral at high velocities, they experience various forces, including centrifugal force, which directs them to specific locations in the fluid stream based on their size. This allows for precise sorting through separate outlets.
To enhance the efficiency of the sorting process, researchers found that running the sorter twice—first at a lower speed to primarily extract larger cells and then at a higher speed to extract the remaining larger cells—greatly improved outcomes, removing about 50 percent of the larger, potentially tumorigenic cells in just one pass. Experimental results confirmed the removed cells correlated with a higher tumor risk.
“While we cannot completely eliminate these cells, we believe this technique will substantially decrease the associated risks. Providing that the original cell population is largely differentiated, this process can significantly enhance the safety of these therapies,” Han states.
Crucially, the microfluidic sorter operates without the need for filters, which can often become clogged or damaged over time. This filter-free design ensures a longer operational lifespan. With initial successes demonstrated, the research team is now moving to conduct larger-scale studies and animal trials to evaluate the efficacy of the purified cells within living organisms.
Eliminating undifferentiated cells can not only mitigate tumor risks but also improve overall treatment effectiveness. “If we can convincingly demonstrate these advantages in vivo, this technique could have far-reaching applications in cell therapy innovations,” says Han.
This groundbreaking research is supported by the National Research Foundation of Singapore and the Singapore-MIT Alliance for Research and Technology.
Photo credit & article inspired by: Massachusetts Institute of Technology