Since the 1950s, the chemotherapy drug 5-fluorouracil (5-FU) has been a cornerstone in the treatment of various cancers, including blood-related malignancies and those affecting the digestive tract. Although the prevailing thought had been that 5-FU operates primarily by damaging DNA, groundbreaking research from MIT reveals an intriguing new perspective: in cases of colon and gastrointestinal cancers, this drug exerts its lethal effects by disrupting RNA synthesis.
This revelation could revolutionize the treatment landscape for numerous cancer patients. Traditionally, 5-fluorouracil is administered alongside other chemotherapy agents that target DNA, but the recent study highlights that this combination may not yield the hoped-for synergistic effects in colon cancer patients. Instead, researchers propose that pairing 5-FU with drugs that interfere with RNA synthesis may enhance its efficacy against gastrointestinal tumors.
“Our research is the most conclusive evidence to date that RNA incorporation of 5-FU, triggering an RNA damage response, is key to its effectiveness in gastrointestinal cancers,” stated Michael Yaffe, the David H. Koch Professor of Science at MIT, and director of the MIT Center for Precision Cancer Medicine. “While textbooks attribute the drug’s mechanism primarily to DNA effects across all cancer types, our findings emphasize that RNA damage plays a critical role in the effectiveness of 5-FU for certain tumor types, particularly GI cancers.”
Leading this pivotal study, Yaffe aims to design clinical trials that evaluate the combination of 5-fluorouracil with drugs dedicated to amplifying its RNA-targeting properties, thereby improving cancer cell elimination.
The research paper, co-authored by Koch Institute scientist Jung-Kuei Chen and former MIT postdoc Karl Merrick, was recently published in Cell Reports Medicine.
The Surprising Mechanism of Action
5-fluorouracil is frequently employed as a frontline treatment for colon, rectal, and pancreatic cancers, often combined with DNA-damaging agents such as oxaliplatin or irinotecan. This dual approach was assumed to be effective because 5-FU disrupts the synthesis of DNA nucleotides, leading to cell death in the presence of DNA damage. However, Yaffe and his team sought to delve deeper into how these combinations actually impact cancer cell viability.
In their investigation, they tested the combination of 5-FU with oxaliplatin and irinotecan in lab-grown colon cancer cells and were astounded by the findings: instead of enhancing cancer cell death, these drugs often displayed less efficacy than anticipated when combined. According to Yaffe, “It was expected that targeting both DNA breakage and nucleotide production would synergistically promote cancer cell death, but our studies reveal that many combinations were antagonistic, hindering one another’s effects.”
To corroborate their laboratory results, the team collaborated with pharmacology expert Adam Palmer from the University of North Carolina, who analyzed data from colon cancer patients treated with these drug combinations. Palmer’s findings confirmed the lack of synergy in most cases, indicating that the dual therapies do not generally cooperate to benefit patient outcomes.
“This suggests a mismatch between the expected and actual effects of these drugs when given together,” explained Yaffe. “While one drug may be effective for certain patients, another drug in the combination may only be beneficial for others, creating uncertainty in optimal treatment choices.”
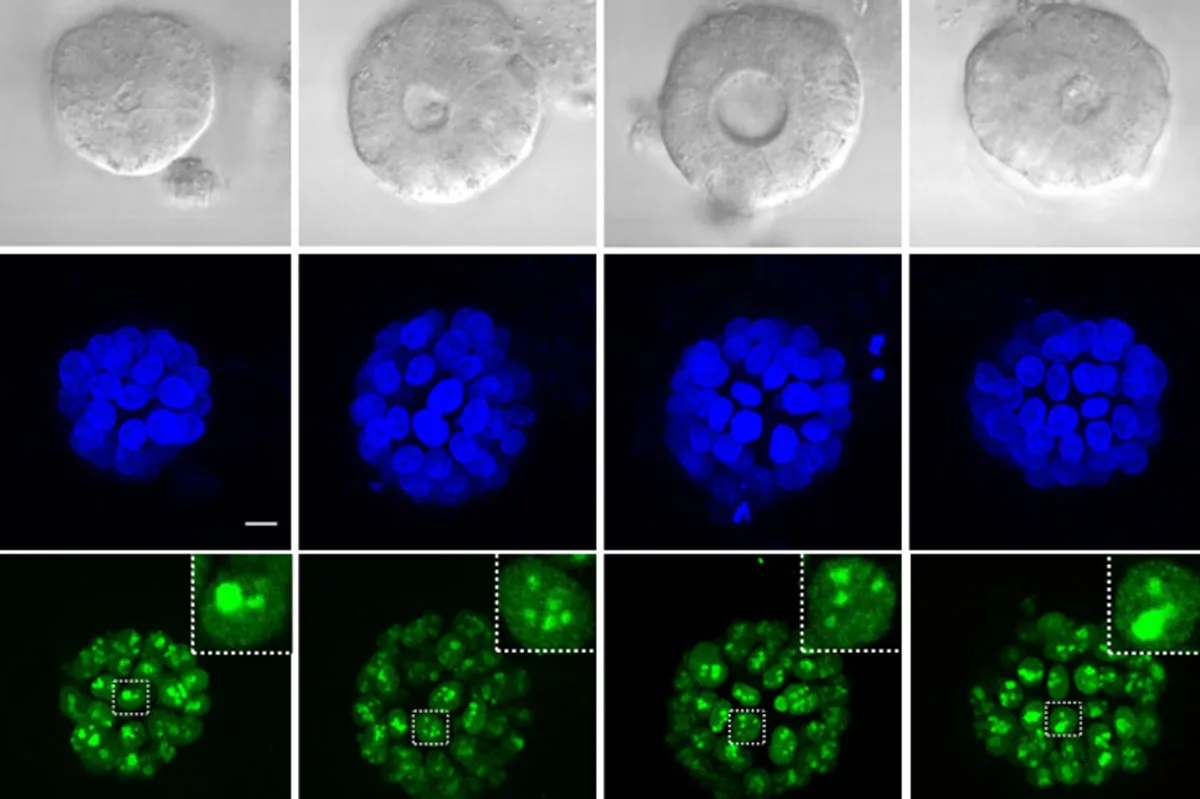
Curious about 5-FU’s actual mechanism of action, the researchers revisited existing studies that indicated the drug’s metabolites also integrate into RNA nucleotides. They discovered that the metabolite affecting RNA was significantly more lethal to colon cancer cells compared to the DNA-disrupting variant.
More specifically, this RNA damage mainly targets ribosomal RNA, the essential component of ribosomes, which are crucial for protein synthesis within cells. Without functioning ribosomes, tumor cells struggle to generate the proteins they need for survival and proliferation, ultimately leading to cell death.
Moving forward, the team is investigating how damage to ribosomal RNA prompts cancer cells to initiate programmed cell death, or apoptosis. They hypothesize that the accumulation of damaged RNAs within structures called lysosomes may trigger this apoptotic signal.
“Our lab aims to decipher the signaling events that occur during disruptions in ribosome production, especially in gastrointestinal and certain ovarian cancers, to understand how these processes are connected to cellular death pathways,” Yaffe noted.
Exploring New Drug Combinations
The insights gained from this research indicate that drugs capable of enhancing ribosome productivity may work synergistically with 5-FU. In their experiments, the researchers discovered that a molecule inhibiting KDM2A, a repressor of ribosome production, significantly increased cancer cell death when coupled with 5-FU treatment.
Additionally, these findings offer an explanation for why drug combinations of 5-FU with DNA-damaging agents often fall short of their potential. Certain DNA-damaging drugs can inhibit ribosome formation, counteracting the effects of 5-FU on RNA. A more promising strategy could involve administering the drugs with a gap between treatments, allowing patients to benefit from each drug’s unique action without interfering with one another.
“Our data don’t imply that current combination therapies are ineffective; we recognize their clinical benefits,” Yaffe clarified. “Instead, our research indicates that minor adjustments in the timing of drug administration could enhance treatment efficacy.”
The researchers plan to collaborate with other institutions to conduct a phase 2 or 3 clinical trial, exploring the effects of a revised drug schedule on patient outcomes.
“A clinical trial is clearly warranted to assess the effectiveness of our findings,” Yaffe stated. “Since these drugs are already established in clinical practice for GI cancers, changing the timing of their administration should be straightforward.”
Moreover, the team hopes this research will identify biomarkers that can forecast which tumors are more vulnerable to 5-FU combinations, potentially exploring RNA polymerase I activity as a candidate biomarker linked to ribosomal RNA production.
This study received funding from the Damon Runyon Cancer Research Fund, the Ludwig Center at MIT Fellowship, the National Institutes of Health, the Ovarian Cancer Research Fund, the Holloway Foundation, and the STARR Cancer Consortium.
Photo credit & article inspired by: Massachusetts Institute of Technology